Iron makes up approximately 8.1% by weight of Earth's crust (Lutgens and Tarbuck 2000) and is naturally found in the form of an ore, most often an iron oxide, such as magnetite (Fe 3O 4) or hematite (Fe 2O 3). The use of steel, an iron‐base alloy, dates far back to the Middle Ages, with the earliest known production of steel dating nearly 4000 years, from 1800 BCE (Akanuma 2005). Iron alloys are generally inexpensive compared to other materials and are highly versatile.
α‐Fe [BCC, Ferrite]
γ‐Fe [FCC, Austenite]
δ‐Fe [BCC]
Pure iron exists in three phases. Below 1185 K, α‐Fe (known as ferrite) has a BCC structure, but it transitions to γ‐Fe (known as austenite) with an FCC structure above this temperature. This transition to a close‐packed structure with an increase in temperature is highly unusual and is the basis of many of the useful properties of steel. Above 1667 K, pure iron transitions back to BCC and is known as δ‐Fe at high temperatures. It is of interest to note that a β‐Fe phase, which was historically thought to exit, was found to be a form of α‐Fe above its Curie temperature (nonmagnetic).
The foundation for understanding the microstructure of steel is the iron‑carbon phase diagram ( Figure 2.15). Iron alloyed with carbon is called carbon steel and has a carbon content that ranges from 0.002 to 2.14% by weight (or from 0.009 to 9.2 mol%). Alloys with a higher carbon content generally have phases of pure carbon graphite within the structure and are known as cast irons. Steels, on the other hand, have low carbon precipitates and benefit from the presence of iron carbides, namely Fe 3C. A key feature of the phase diagram is the transformation, upon cooling, of austenite to an intimate mixture of ferrite and carbide. Thin platelets of Fe 3C become immersed in an α‐Fe matrix in a two‐phase mixture called pearlite, and the interlamellar spacing can be controlled with transformation temperature.
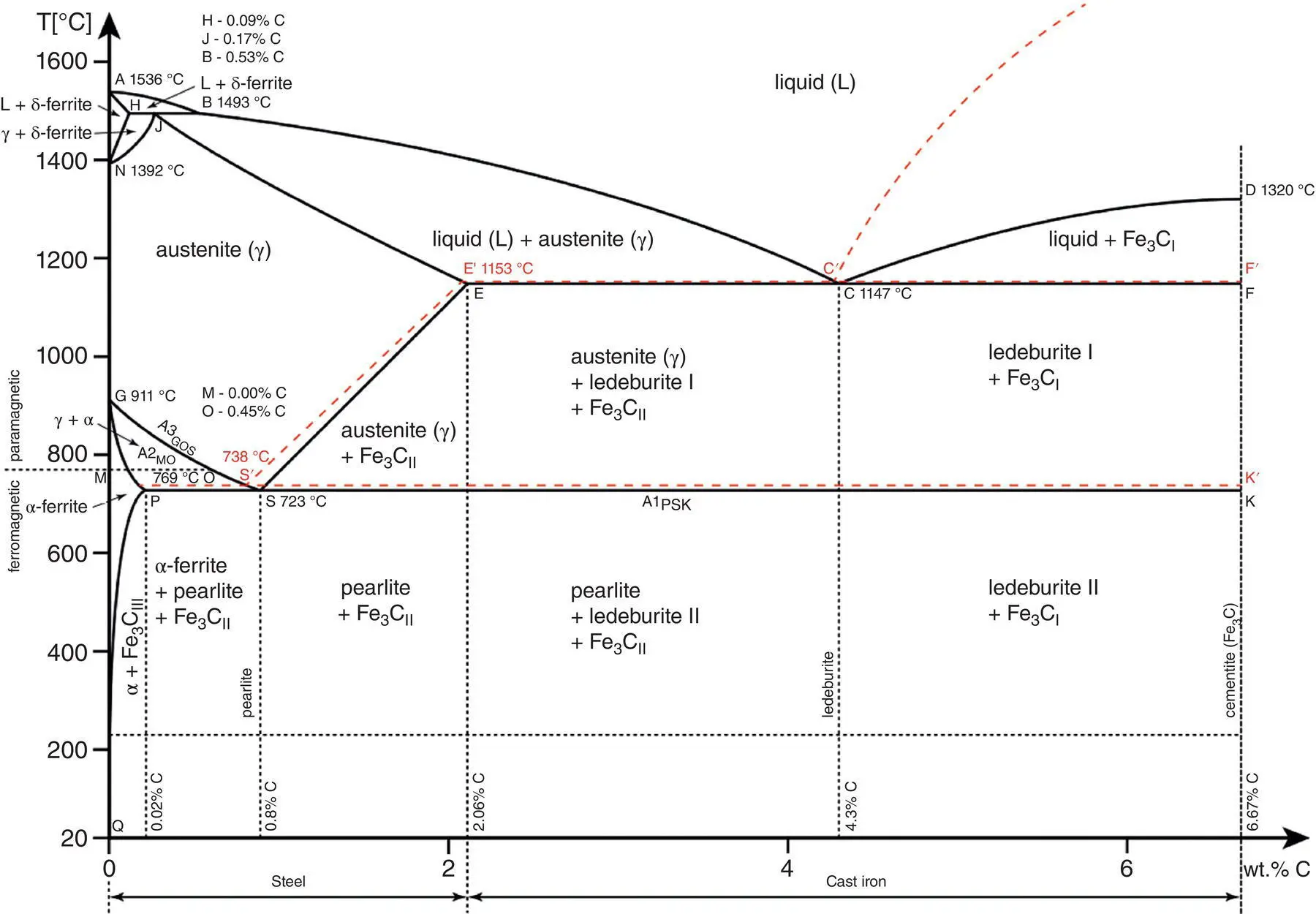
Figure 2.15 Iron‐carbon phase diagram demonstrating the main phases under atmospheric pressure.
Source: Caesar (2019), https://commons.wikimedia.org/wiki/File:Iron_carbon_phase_diagram.svg. Licensed under CC BY‐SA 4.0.
An iron‑carbon phase that is metastable and so does not appear on the phase diagram but is highly important to engineering materials is called martensite. Martensite is a body‐centered tetragonal phase, like BCC, iron, but with an elongated c ‐axis due to the placement of carbon atoms at 0, 0, ½ sites. It is extremely hard and strong, but is also brittle. Tempering of martensite forms a two‐phase mixture of ferrite and carbide with the carbide present as small particles rather than platelets. Tempered martensite is stronger and tougher than either pearlite or martensite. Specialized heat treatment of the iron‐carbide system can produce other mixtures, such as bainite (a plate‐like mixture where the carbide within the plates is particulate rather than platelet like) and a spheroidite (nearly spherical iron carbide). Each mixture can give rise to different properties.
The majority of steels are not plain carbon steels. Rather, they are alloyed steels that are modified to obtain different properties with the addition of alloying elements, such as manganese, nickel, chromium, molybdenum, boron, titanium, vanadium, tungsten, cobalt, and niobium. For example, stainless steels contain a minimum of 11% chromium in order to resist corrosion through the formation of a self‐repairing (in the presence of oxygen) passive layer of chromium oxide on the surface. Chromium addition with little to no nickel produces ferritic (BCC) stainless steel similar to carbon steel. Nickel is added to stainless steels to form austenitic stainless steels. Nickel (as well as manganese) helps stabilize the austenitic (FCC) structure and results in high toughness and high strength throughout a broad temperature range. For example, grade 316 stainless steel nominally contains (in wt.%) 17 Cr, 12 Ni, 2 Mn, 1 Si, 0.1 C, and 2.5 Mo with balance Fe (Carter and Paul 1991).
Stainless steel can also be precipitation hardened to increase strength and corrosion resistance. The composition of such material can become quite complex. For example, Discaloy is a common precipitation‐hardened stainless steel with a nominal composition (in wt.%) of 26 Ni, 13.5 Cr, 2.75 Mo, 1.75 Ti, 0.90 Mn, 0.80 Si, 0.08 C, and 0.005 B with balance Fe.
The complexity of iron‐base alloys has enabled them to be remarkably versatile. However, as the boundaries to high‐temperature use are pushed even higher, the search for alternative materials has led to remarkable superalloy materials outside of the iron system.
Nickel is the fifth most abundant element on Earth, but most of it is located almost 3000 km below the surface (United States Geological Survey 2012). In Earth's crust, magmatic sulfide deposits (principal ore mineral pentlandite, (Ni,Fe) 9S 8) and laterite deposits (ore mixtures of nickeliferous limonite, (Fe,Ni)O(OH), and garnierite – a mixture of hydrous nickel and nickel‐rich silicates) (United States Geological Survey 2012). Roughly 3000 nickel‐base alloys are in use, forming products for numerous industries, including energy, chemical, petrochemical, and aerospace industries. Ni‐base systems have risen as systems of choice for tailored superalloys largely due to the nature of the precipitates and resulting properties that can be achieved in the system. As such, a deeper look into nickel‐base alloys is critical to frame later discussion in the text.
Nickel‐base alloys tend to have a fully austenitic (FCC) structure and the ability to maintain good tensile, rupture, and creep properties to much higher temperatures than (BCC) systems. They are often used in load‐bearing structures and nickel‐base superalloys have been reported being used to highest homologous temperature (up to T m= 0.9) of any common alloy system (Bowman 2000). Like Fe‐base alloys, the properties of Ni‐base alloys can be tailored through the addition of many other elements, including chromium, iron, cobalt, molybdenum, tungsten, tantalum, aluminum, titanium, zirconium, niobium, rhenium, yttrium, vanadium, carbon, boron, and hafnium. Nickel‐base alloys can be solid‐solution or precipitation strengthened, but the latter is generally used for more demanding applications.
The properties of Ni‐base superalloys have largely arisen through their unique phases and the ability to tailor the properties through the addition of various elements. Ni‐base superalloys are generally composed of a gamma (γ) phase, which forms an austenitic solid‐solution matrix composed of the alloying elements. As the alloys are cooled from the melt, gamma prime (γ′) phases begin to precipitate within the γ‐matrix ( Figure 2.16) (Betteridge and Heslop 1974; Ross and Sims 1987). γ′ phase is a GCP phase generally composed of Ni 3(Al,Ti) in Ni‐base superalloys. It is the main strengthening phase in the alloy and has strong coherency with the matrix, which allows for ductility. The precipitates can take on several different geometries, including cuboidal, spheres, platelets, or combinations thereof. However, to decrease their energy states, they often align along the <100> directions and form cuboidal structures (Sabol 1969). Many Ni‐base superalloys can thus be described as having ordered γ′ particles within a disordered γ‐matrix.
The γ–γ′ system forms the basic structure of Ni‐base superalloys, but several other phases can also be present:
Читать дальше













