John O'Brien - Earth Materials
Здесь есть возможность читать онлайн «John O'Brien - Earth Materials» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Earth Materials
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:5 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 100
- 1
- 2
- 3
- 4
- 5
Earth Materials: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Earth Materials»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
Earth Materials,
Earth Materials,
Earth Materials — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Earth Materials», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
Figure 4.14 Crystallographic axes (positive ends labeled) and intersection angles for the major crystal systems: isometric, tetragonal, orthorhombic, monoclinic, triclinic, and hexagonal systems.
4.5.2 Crystal forms
Each of the crystal systems has an associated set of common (and rarer) crystal forms. Crystal formsconsist of a three‐dimensional set of one or more crystal faces that possess similar spatial relationships to the crystallographic axes. Some natural crystals possess only one crystal form; others possess multiple or combined crystal forms. Crystal forms can be subdivided into two major groups: closed forms and open forms.
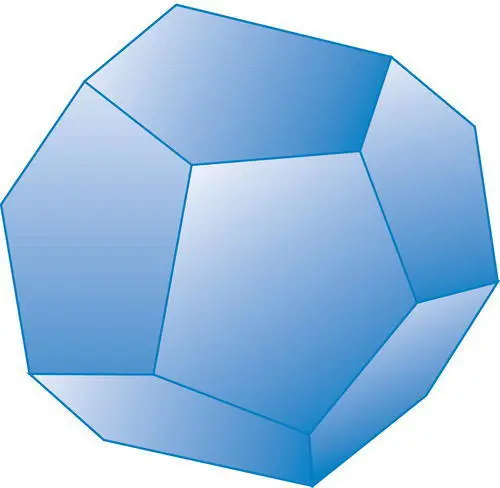
Figure 4.15 A pyritohedron, a closed form in which all faces have the same general relationship to the crystallographic axes.
Closed crystal formscan completely enclose a mineral specimen and therefore can exist alone in perfectly formed (euhedral) crystals which are enclosed completely by the crystal form. Common closed forms include all the forms in the isometric system and many forms in the tetragonal, hexagonal, trigonal, and orthorhombic systems. The pyritohedron ( Figure 4.15) is a typical closed form, common in the mineral pyrite. Each closed form possesses a different shape that is related to the number and shape of faces in the form and to their angular relationships to the crystallographic axes. Figure 4.16illustrates common dipyramid (double pyramid) closed forms in the trigonal, tetragonal, and hexagonal systems.
Open crystal forms( Figure 4.17) cannot completely enclose a mineral specimen and so must occur in combination with other open or closed crystal forms in order to completely enclose a crystal. Common open forms include: (1) pedions, which consist of a single face, (2) pinacoids, a pair of parallel faces, (3) prisms, three or more faces parallel to an axis, (4) pyramids, three or more faces that intersect an axis, (5) domes, a pair of faces symmetrical about a mirror plane, and (6) sphenoids, a pair of faces symmetrical about an axis of rotation. Figure 4.17b illustrates the kinds of prisms that occur in the trigonal, tetragonal, and hexagonal crystal systems.
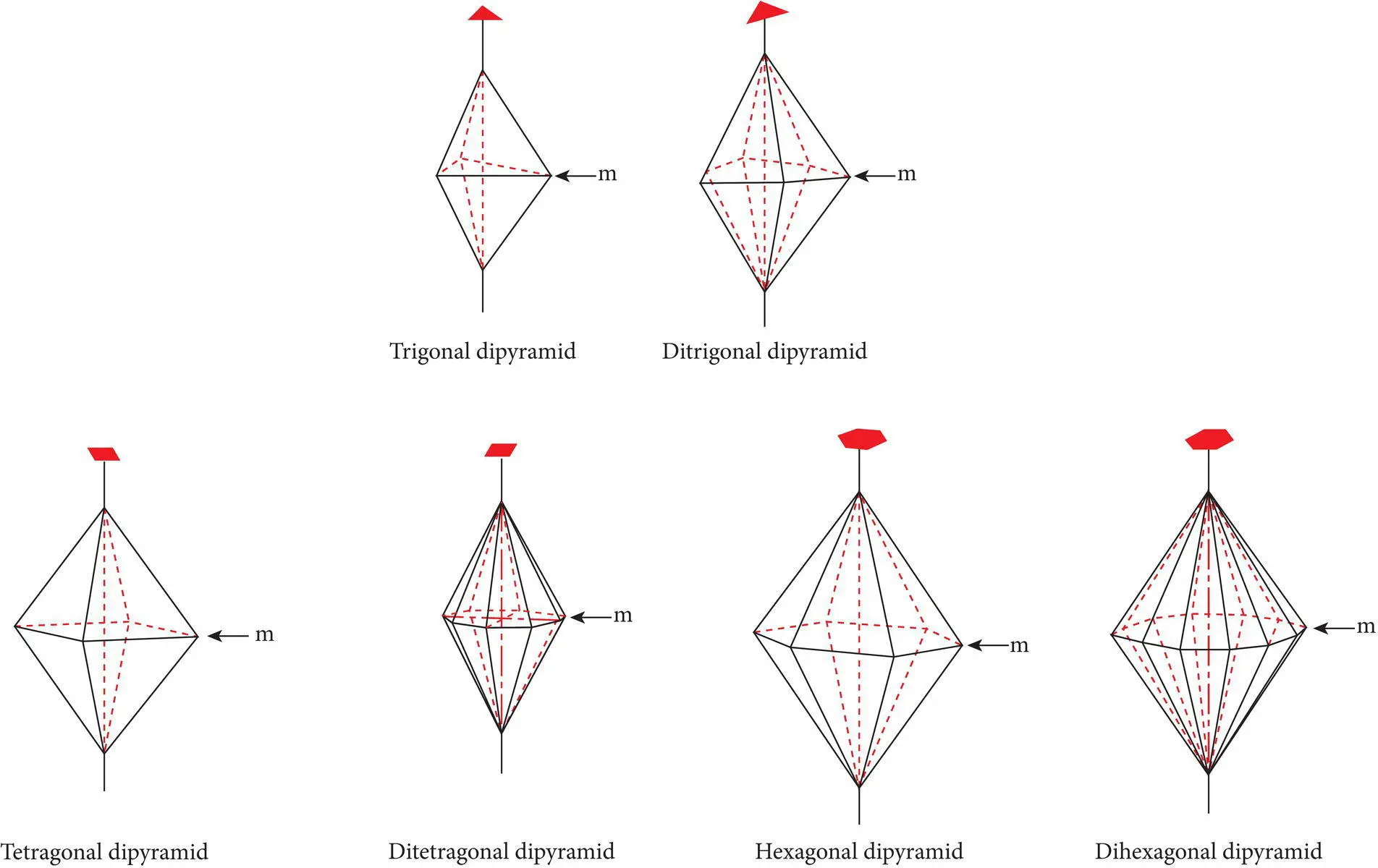
Figure 4.16 Different types of dipyramid forms in the trigonal, tetragonal, and hexagonal systems.
Source : Klein and Hurlbut (1985). © John Wiley & Sons.
The most common crystal forms in each system are discussed later in this chapter, after we have presented the language used to describe them. More detailed discussions are available in Nesse (2016) and Klein and Dutrow (2007).
4.6 INDEXING PLANES IN CRYSTALS
4.6.1 Axial ratios
Whatever their respective lengths, the proportional lengths or axial ratiosof the three crystallographic axes (a : b : c) can be calculated. The standard method for expressing axial ratios is to express their lengths relative to the length of the b‐axis (or a 2‐axis) which is taken to be unity so that the ratio is expressed as a : b : c; b = 1. This is accomplished by dividing the lengths of all three axes by the length of the b‐axis (a/b: b/b: c/b). An example from the monoclinic system, the pseudo‐orthorhombic mineral staurolite, will illustrate how axial ratios are calculated. In staurolite, the unit cell edges have average dimensions expressed in angstrom (Å) units of: a = 7.87 Å, b = 16.58 Å, and c = 5.64 Å. The axial ratios are calculated from a/b : b/b : c/b = 7.87/16.58 Å : 16.58/16.58 Å : 5.64/16.58 Å. The average axial ratios of staurolite are 0.47 : 1.00 : 0.34.
Axial ratios are essential to understanding how crystallographic planes and crystal forms are described or indexed by reference to the crystallographic axes as discussed in the section that follows.
4.6.2 Crystal planes and crystallographic axes
Crystalline substances such as minerals have characteristic planar features that include: (1) crystal faces that develop during growth, (2) cleavage surfaces that develop during breakage, and (3) crystal lattice planes that reflect X‐rays and other types of electromagnetic radiation. All these types of planes possess a number of shared properties.
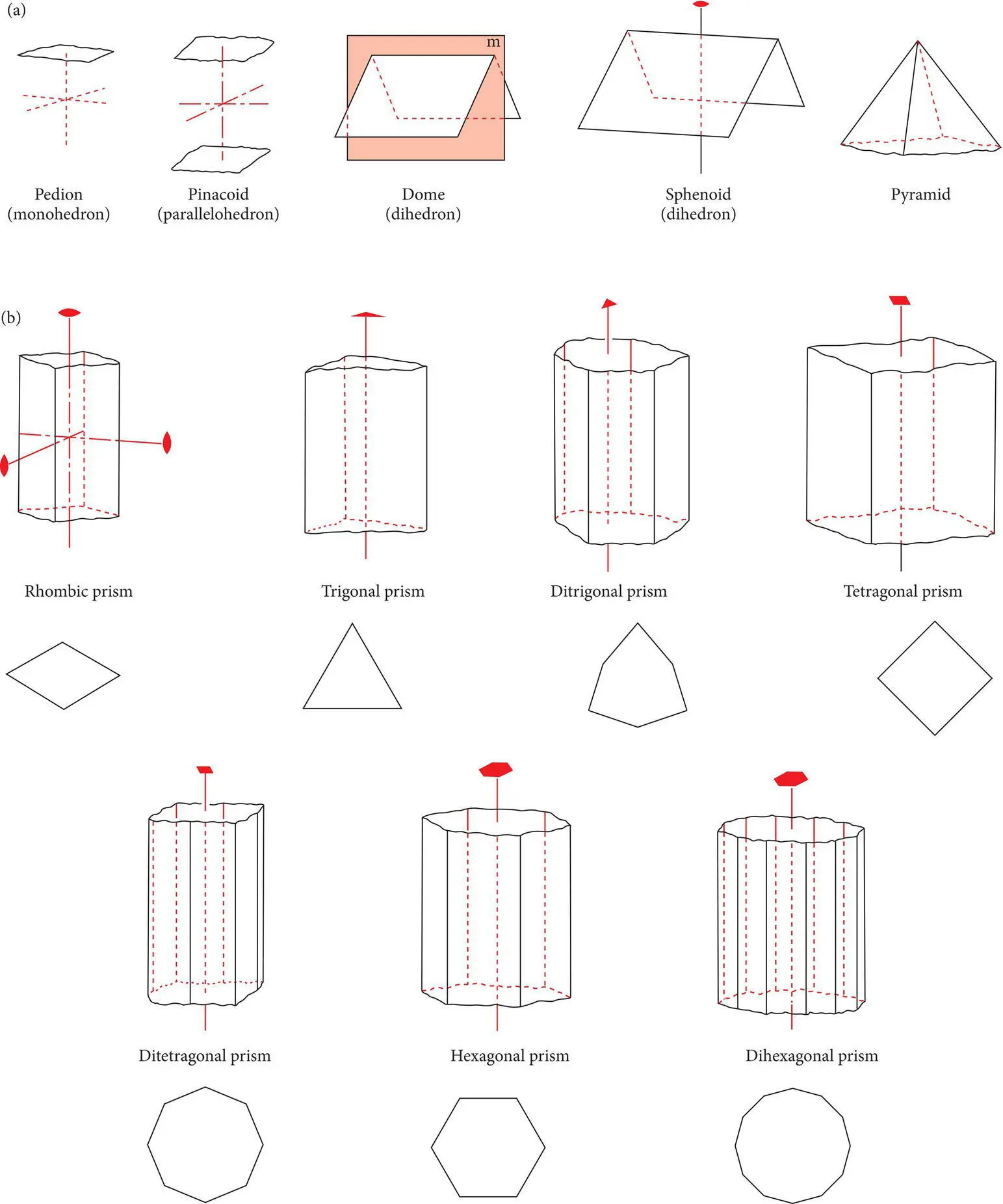
Figure 4.17 (a) Common open forms: pedions, pinacoids, domes, sphenoids, and pyramids. (b) Different types of prisms that characterize the orthorhombic, trigonal, tetragonal, and hexagonal systems. The illustrated prisms are bounded by pinacoids at the top and bottom.
Source : Klein and Hurlbut (1985). © John Wiley & Sons.
Each type of plane is part of a large set of parallel lattice planes of which it is representative. As a mineral with a particular crystal form grows freely it may be bounded by a sequence of planar faces. When it stops growing, it is bounded by crystal faces that are parallel to many other lattice planes that bounded the mineral as it grew over time. When a mineral cleaves, it breaks along a specific set of parallel planes of relative weakness, but these cleavage planes are parallel to large numbers of planes of weakness or potential cleavage surfaces in the mineral structure along which the mineral did not happen to rupture. When X‐rays are reflected from a reflecting plane, they are reflected simultaneously from all the planes in the crystal that are parallel to one another to produce a “reflection peak” that is characteristic of the mineral and can be used to identify it.
In addition, any set of parallel planes in a crystal is ideally characterized by a particular molecular content; all the parallel planes in the set possess closely similar molecular units, spacing, and arrangement. A molecular image of one of these planes is sufficient to depict the general molecular content of all the planes that are parallel to it. All planes in a set of parallel planes have the same general spatial relationship to the three crystallographic axes. This means that they can be collectively identified in terms of their spatial relationship to the three crystallographic axes. This is true for crystal faces, for cleavage surfaces, for X‐ray reflecting planes or for any set of parallel crystallographic planes that we wish to identify. A universally utilized language has evolved that uses the relationship between the planar features in minerals and the crystallographic axes to identify different sets of planes. A discussion of this language and its use follows.
Figure 4.18depicts several representative crystal planes with different relationships to the three crystallographic axes. Some crystal planes, or sets of parallel planes, intersect one crystallographic axis and are parallel to the other two ( Figure 4.18a, b). Alternatively, a set of crystal planes may intersect two crystallographic axes and be parallel to the third ( Figure 4.18c, d). Still other sets of planes intersect all three crystallographic axes ( Figure 4.18e, f). No other possibilities exist in Euclidean space; sets of planes in crystalline substances must intersect one, two, or three axes and be parallel to those they do not intersect.
Читать дальшеИнтервал:
Закладка:
Похожие книги на «Earth Materials»
Представляем Вашему вниманию похожие книги на «Earth Materials» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Earth Materials» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.












