John O'Brien - Earth Materials
Здесь есть возможность читать онлайн «John O'Brien - Earth Materials» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Earth Materials
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:5 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 100
- 1
- 2
- 3
- 4
- 5
Earth Materials: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Earth Materials»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
Earth Materials,
Earth Materials,
Earth Materials — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Earth Materials», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
Table 3.2 The 14‐step radioactive decay sequence that occurs in the conversion of the radioactive isotope 238U into the stable isotope 206Pb.
| Parent isotope | Daughter isotope | Decay process | Half‐life |
|---|---|---|---|
| Uranium‐238 | Thorium‐234 | Alpha | 4.5 × 10 9years |
| Thorium‐234 | Protactinium‐234 | Beta | 24.5 days |
| Protactinium‐234 | Uranium‐234 | Beta | 1.1 minutes |
| Uranium‐234 | Thorium‐230 | Alpha | 2.3 × 10 5years |
| Thorium‐230 | Radium‐226 | Alpha | 8.3 × 10 4years |
| Radium‐226 | Radon‐222 | Alpha | 1.6 × 10 3years |
| Radon‐222 | Polonium‐218 | Alpha | 3.8 days |
| Polonium‐218 | Lead‐214 | Alpha | 3.1 minutes |
| Lead‐214 | Bismuth‐214 | Beta | 26.8 minutes |
| Bismuth‐214 | Polonium‐214 | Beta | 19.7 minutes |
| Polonium‐214 | Lead‐210 | Alpha | 1.5 × 10 −4seconds |
| Lead‐210 | Bismuth‐210 | Beta | 22.0 years |
| Bismuth‐210 | Polonium‐210 | Beta | 5.0 days |
| Polonium‐210 | Lead‐206 | Alpha | 140 days |
Box 3.2Radon and lung cancer
Inhalation of radon gas is the second largest cause of lung cancer worldwide, second only to cigarette smoking. In the 1960s, underground uranium miners began to show unusually high incidences of lung cancer. The cause was shown to be related to the duration of the miner's exposure to radioactive materials. To cause lung cancer, the radioactive material must enter the lungs as a gas. It then causes progressive damage to the bronchial epithelium or lining of the lungs. What is the gas and how does it originate? Table 3.2shows the many radioactive isotopes that are produced by the decay of the common isotope of uranium ( 238U). Uranium miners would be exposed to all of these, but which one would they inhale into their lungs? Because radon possesses a stable electron configuration, it tends not to combine with other elements. Like most noble elements, under normal near surface conditions, it tends to exist as separate atoms in the form of a gas. In the confined space of poorly ventilated underground mines, radioactive decay in the uranium series produces sufficient concentrations of radon to significantly increase the incidence of lung cancer. The other property that makes radon‐222 so dangerous is its short half‐life (3.825 days). Within days, most of the radon inhaled by miners decays into polonium‐218 with the emission of alpha particles ( 4He nuclei). Subsequently, most of the radioactive 218Po decays within hours into lead‐210 with the release of more alpha particles. Lung damage leading to lung cancer largely results from continued rapid release of alpha particles over long periods of exposure. Scientific studies on radon exposure have been complicated by the fact that many miners were also smokers. It turns out that smoking and radon exposure act synergistically to multiply the risk of developing lung cancer.
Is the general public at risk of radon exposure? Uranium is ubiquitous in the rocks of Earth's crust, and so therefore is radon production. Potassium feldspar‐bearing rocks such as granites and gneisses, black shales, and phosphates contain higher uranium concentrations (>100 ppm) than average crustal rocks (<5 ppm). They therefore pose a greater threat. Radon gas occurs in air spaces and is quite soluble in water; think of the dissolved oxygen that aqueous organisms use to respire or the carbon dioxide dissolved in carbonate beverages. Groundwater circulating through uranium‐rich rocks can dissolve substantial amounts of radon gas and concentrate radium, another carcinogenic isotope. Ordinarily, this is not a problem. The gas rises and is released to the atmosphere, where it is dispersed and diluted to very low levels. But if radon gas is released into a confined space such as a home, especially one that is well insulated and not well ventilated, radon gas concentrations can reach hazardous levels. Most radon gas enters the home through cracks in the walls and foundations, either as gas or in water from which the gas is released. Most of the remainder is released when water from radon‐contaminated wells is used, again releasing radon into the home atmosphere. The problem is especially bad in winter and spring months when homes are heated, basements flooded, and ventilation poor. As warm air in the home rises, air is drawn from the soil into the home, increasing radon concentrations. The insulation that increases heat efficiency also increases radon concentrations. What can be done to reduce the risk? Making sure that basements and foundation walls are well sealed and improving ventilation can reduce radon concentrations to acceptable levels, even in homes built on soils with high concentrations of uranium. Radon test kits can be purchased from hardware stores. If indoor radon levels exceed 4 pCi/l, remediation is recommended by the installation of indoor air pumps and ventilation pipes to remove gases from beneath basement floors. Radon remediation typically costs $1500 and is highly recommended as a health measure.
In the following sections we have chosen a few examples, among the many that exist, to illustrate the importance of radioactive isotopes and decay series in the study of Earth materials.
Age determinations using radioactive decay series
Table 3.3lists several radioactive parent to stable daughter transformations that can be used to determine the formation ages of Earth materials. All these are based on the principle that after the radioactive isotope is incorporated into Earth materials, the ratio of radioactive parent isotopes to stable daughter isotopes decreases through time by radioactive decay. The rate at which such parent: daughter ratios decrease depends on the rate of decay , which is given by the decay constant (λ) , the proportion of the remaining radioactive atoms that will decay per unit of time. One useful formula that governs decay series states that the number of radioactive atoms remaining at any given time (N) is equal to the number of radioactive atoms originally present in the sample (N 0) multiplied by a negative exponential factor (e –λt) that increases with the rate of decay ( λ) and the time since the sample formed (t), that is, its age. These relationships are given by:
Table 3.3 Systematics of radioactive isotopes important in age determinations in Earth materials.
| Decay series | Decay process | Decay constant (λ) | Half‐life | Applicable dating range |
|---|---|---|---|---|
| 14C → 14N | Beta decay | 1.29 × 10 −4/year | 5.37 Ka | <60 Ka |
| 40K → 40Ar | Electron capture | 4.69 × 10 −10/year | 1.25 Ga | 25 Ka to >4.5 Ga |
| 87Rb → 87Sr | Beta decay | 1.42 × 10 −11/year | 48.8 Ga | 10 Ma to >4.5 Ga |
| 147Sm → 143Nd | Alpha decay | 6.54 × 10 −12/year | 106 Ga | 200 Ma to >4.15 Ga |
| 232Th → 208Pb | Beta and alpha decays | 4.95 × 10 −11/year | 14.0 Ga | 10 Ma to >4.5 Ga |
| 235U → 207Pb | Beta and alpha decays | 9.85 × 10 −10/year | 704 Ma | 10 Ma to >4.5 Ga |
| 238U → 206Pb | Beta and alpha decays | 1.55 × 10 −10/year | 4.47 Ga | 10 Ma to >4.5 Ga |
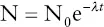
It should be clear from the formula that when t = 0, N = N 0, and that N becomes smaller through time as a function of the rate of decay given by the decay constant; rapidly for a large decay constant, more slowly for a small one. Figure 3.13illustrates a typical decay curve, showing how the abundance of the radioactive parent isotope decreases exponentially over time while the abundance of the daughter isotope increases in a reciprocal manner. The two curves cross where the number of radioactive parent and stable daughter atoms is equal. The time required for this to occur is called the half‐life of the decay series and is the time required for one half of the radioactive isotopes to decay into stable daughter isotopes.
Читать дальшеИнтервал:
Закладка:
Похожие книги на «Earth Materials»
Представляем Вашему вниманию похожие книги на «Earth Materials» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Earth Materials» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.












