Role of implant surfaces on implant stability
Any given implant geometry surfaces prepared with a microrough topography are considerably more osteoconductive compared with the original machined-surface implants 20, 21(see Fig 1-1). There are several reasons why these surfaces are such an improvement over the original machined surfaces. First, the modern implant surfaces with microrough surface topographies retain the fibrin blood clot more effectively than implants with machined surfaces. 22As a result, the initial critical events (ie, plasma protein adsorption, clot formation, angiogenesis, local stem cell and repair cell migration and attachment, cell differentiation) associated with osseointegration are facilitated.
In addition, local stem cells differentiate more rapidly into functioning osteoblasts following attachment to the microrough surfaces as compared with machined surfaces. These surfaces also upregulate and accelerate the expression of genes of the differentiating osteoblasts associated with the osseo- integration process. 23This leads to a different combination of collagenous and noncollagenous proteins making up the bone deposited on the microrough surfaces as compared with the bone deposited on machined-surface topographies. As a result, bone that matures on implant surfaces with microrough surface topography is harder and stiffer than bone deposited on machined surfaces. 24, 25
An active and efficient remodeling apparatus is key to maintaining osseointegration during functional loading of the implants. 26Osseointegration of the implant with bone continues to occur up to 1 year following delivery of either a provisional or definitive prosthesis. 27Following initial healing and functional loading within physiologic limits, progressive osteogenesis continues to where the bone-implant contact area approaches almost 90% in favorable sites ( Fig 1-6).
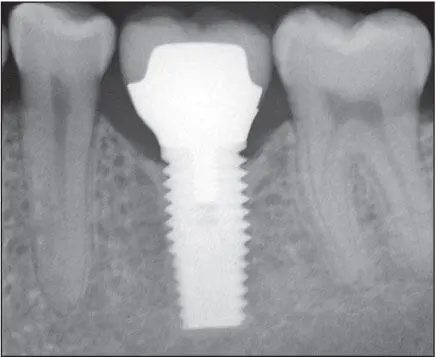
Fig 1-6 Following initial healing and when loading forces are favorable, the bone contact area on the surface of the implant continues to increase. Note the bone density of the peri- implant bone 7 years following delivery.
The Implant–Soft Tissue Interface
The peri-implant mucosa is similar to the mucosa circumscribing natural teeth. It is composed of nonkeratinizing epithelium in the sulcus, junctional epithelium, and a supracrestal zone of connective tissue. The connective tissue layer contains a dense zone of circumferential collagen fibers intermingled with fibers extending outward from the alveolar crest. These fibers run parallel to the long axis of the implant. The zone of connective tissue adjacent to the implant is relatively avascular and acelluar and similar to scar tissue histologically. The soft tissue barrier (interface) assumes a minimal dimension during the healing process. If this dimension is less than 2 to 3 mm, bone resorption occurs in order to establish an appropriate biologic dimension of the peri-implant soft tissue barrier. 28
The titanium–soft tissue interface appears to be similar to but not exactly the same as that seen between gingiva and natural dentition ( Fig 1-7). The epithelial-implant interface is based on the hemidesmosome basal lamina system, similar to that seen between gingiva and teeth. When implants emerge through attached keratinized mucosa, collagen fibers circumferentially configured around the neck of the implant are interwoven with collagen fibers running from the crest of the alveolus and the periosteum to the free gingiva and hold the epithelium in close proximity to the surface of the implant. The epithelial cells in the sulcus epithelium secrete a sticky substance (a protein network of glycoproteins) onto the surface of the implants, enabling the epithelial cells to adhere to the implant surface via hemidesmosomes. The epithelial cuffs that form as a result of the basal lamina hemidesmosomal system and the zone of connective tissue just apical to it effectively seal the bone from oral bacteria. 29However, what differentiates the soft tissues around implants from the gingival tissues around natural teeth is the absence of gingival fibers inserting into a cementumlike tissue. Hence, the soft tissues around implants are more easily detached from the surfaces of the implant than are the soft tissues surrounding natural teeth. This difference is clinically significant for a number of reasons, including the manner in which these tissues respond to the oral microflora, 29and especially when cement systems are used for retention of implant prostheses because of the risk of embedding cement subgingivally during cementation of the prosthesis 30thereby increasing the risk of peri-implantitis 31( Fig 1-8).
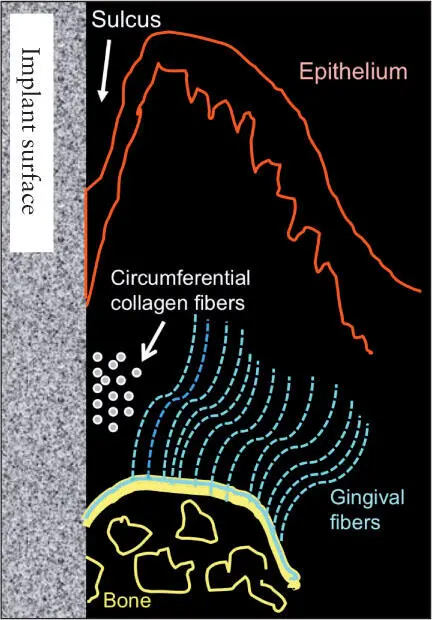
Fig 1-7 Soft tissue–implant interface.
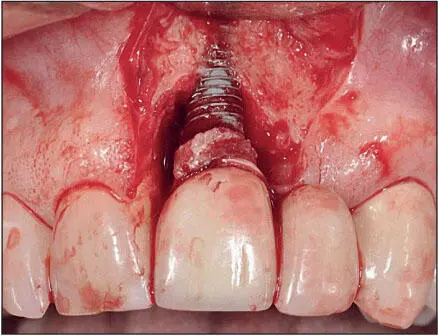
Fig 1-8 Peri-implantitis triggered by excess cement beneath the peri-implant soft tissues. The bone loss has compromised the periodontal support of the adjacent teeth. (Reprinted from Moy et al 3with permission.)
The phenomenon of biologic width applies not only to the natural dentition but also to the soft tissues around implants. Biologic width is defined as the combined length of the supracrestal connective tissue and the zone of junctional epithelium associated with the epithelial attachment 32( Fig 1-9). This dimension averages approximately 3 mm around implants 28and is slightly greater than that associated with the natural dentition. In general, the width of the epithelial component is greater and demonstrates more variability than the width of the connective tissue zone. This phenomenon has particular impact in the esthetic zone because, as with the natural dentition, the level and contours of the underlying bone primarily determine the contours and level of the overlying soft tissues. The zonal epithelium can be located on either the implant fixture or the abutment, depending whether the implant platform is supracrestal, crestal, or subcrestal. The dimension of the biologic width in relation to the nature and topography of the implant surface has been the subject of much debate in recent years. However, there is no clear consensus on whether differences in biologic width exist with respect to the varieties of surface topographies and surface treatments currently in use. 33Also, the evidence appears to indicate that there are no significant differences in biologic width achieved between one-stage and two-stage surgical procedures.
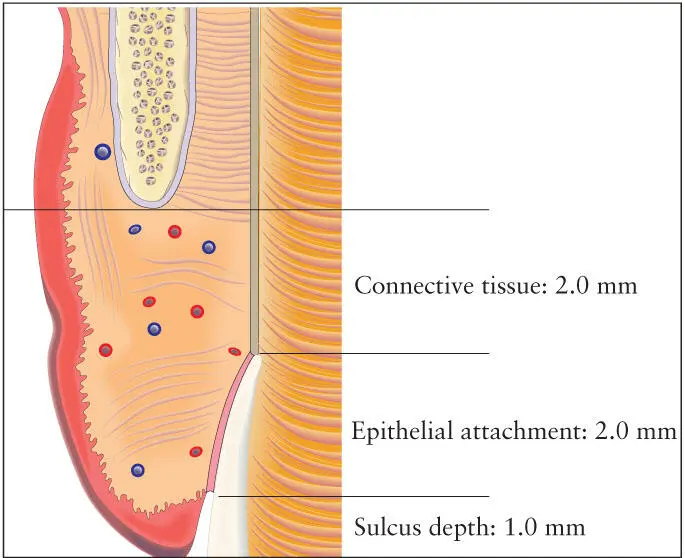
Fig 1-9 Biologic width is defined as the combined length of the supracrestal connective tissue and the zone of junctional epithelium associated with the epithelial attachment. (Redrawn from Spear 32with permission.)
However, it appears that the nature of the microgap between the abutment and the implant and its position in relation to the bone crest increases the biologic width. The deeper the implant-abutment connection in relation to the gingival crest, the greater the biologic width will be, particularly the epithelial component. It is unclear whether multiple abutment manipulations induce an apical migration of the connective tissue–epithelial attachment zone, resulting in marginal bone loss. 34, 35The lack of stability of the abutment-implant connection may also trigger an apical migration of the connective tissue–epithelial attachment zone accompanied by marginal bone loss around the neck of the implant, presumably as a result of increased levels of bacterial colonization. The long-term clinical consequences of these findings with respect to implant survival have yet to be determined.
Читать дальше