Clinical Associate
American University of Beirut Medical Center
Private Practice
Beirut, Lebanon
Chapter 18
Donald R. Schwass, BDS, DClinDent
Clinical Director
Faculty of Dentistry
University of Otago
Dunedin, New Zealand
Chapter 3
Pravej Serichetaphongse, DDS, MS
Associate Professor
Department of Prosthodontics
Chair of the Esthetic Implant Program
Head of the Maxillofacial Prosthetics Unit
Faculty of Dentistry
Chulalongkorn University
Bangkok, Thailand
chapters 3, 12, and 13
Kumar C. Shah, BDS, MS
Professor of Clinical Dentistry
Director of Residency in Advanced Prosthodontics
Division of Advanced Prosthodontics
University of California, Los Angeles
Los Angeles, California
chapters 1, 3, 4, 5, 8, 11, 12, 14, and 19
Arun B. Sharma, BDS, MSc
Clinical Professor of Health Sciences
Director of Graduate Prosthodontics
Division of Preventive and Restorative Sciences
School of Dentistry
University of California, San Francisco
San Francisco, California
Chapter 16
Eric Sung, DDS
Professor of Clinical Dentistry
Vice Chair
Division of Advanced Prosthodontics
School of Dentistry
University of California, Los Angeles
Los Angeles, California
Chapter 17
Andrew Tawse-Smith, DDS, CPerio, PhD
Associate Professor, Periodontics
Associate Dean, International
Department of Oral Sciences
Faculty of Dentistry
University of Otago
Dunedin, New Zealand
chapters 2 and 20
Darryl C. Tong, BDS, MBChB, MSD, PhD
Professor of Oral and Maxillofacial Surgery
He ad of the Department of Oral Diagnostic and Surgical Sciences
Faculty of Dentistry
University of Otago
Dunedin, New Zealand
Chapter 17
Neil Waddell, HDE, PGDipCDTech, MDipTech, PhD
Professor and Head
Discipline of Biomaterials
Department of Oral Rehabilitation
Faculty of Dentistry
University of Otago
Dunedin, New Zealand
Chapter 4
Chandur Wadhwani, BDS, MSD
Affiliate Assistant Professor
Department of Restorative Dentistry
School of Dentistry
University of Washington
Seattle, Washington
Private Practice
Bellevue, Washington
chapters 12, 13, and 14
Benjamin M. Wu, DDS, PhD
Professor and Chair
Division of Advanced Prosthodontics
Di rector of the Jane and Jerry Weintraub Center for Reconstructive Biotechnology
Ex ecutive Director of the Innovative Digital Dentistry Systems
School of Dentistry
Professor
Departments of Bioengineering and Materials Science
School of Engineering
Professor
Department of Orthopedic Surgery
School of Medicine
University of California, Los Angeles
Los Angeles, California
chapters 1, 2, 3, 4, 5, 8, 11, and 12
I
Foundational Principles
CHAPTER 1
History and Biologic Foundations
John Beumer III | Robert F. Faulkner | Kumar C. Shah | Benjamin M. Wu
Introduction and Historical Perspectives
Osseointegration has had a greater impact on the practice of dentistry than any technology introduced during the last 60 years. Since the introduction of osseointegrated dental implants more than 30 years ago, significant advances have been achieved in implant surface bioreactivity, methods used in diagnosis and treatment planning—particularly 3D imaging, computer-aided design (CAD), computer-aided manufacturing (CAM), additive manufacturing, and surface engineering—enhancement of bone and soft tissues of potential implant sites, and prosthodontic approaches and techniques. A degree of predictability with implants has been achieved that is truly remarkable.
The concept of osseointegrated implants was first introduced by Brånemark. 1These implants were made of titanium, and when placed in the jaws, bone was deposited on their surfaces, firmly anchoring the implants in the surrounding bone 1– 3( Fig 1-1). This phenomenon was discovered quite by accident. In a series of experiments designed to document bone healing in vivo, Brånemark used an optical chamber made of titanium placed in a rabbit tibia that was connected to a microscope. When he attempted to remove the chamber from its bone site, he noticed that the bone adhered to the titanium chamber with great tenacity. He recognized the importance of this discovery, and during the next several years, he experimented with various sizes and shapes of dental implants, testing more than fifty designs. He and his colleagues finally settled on a simple screw shape with a hex at the top.

Fig 1-1 The gap between the wall of the osteotomy and the surface of the implant is filled with bone by means of contact (arrows) and distance osteogenesis. (Reprinted from Moy et al 3with permission.)
Most of the previous implant systems were made of cobalt-chrome alloys and were subject to corrosion and release of metallic ions into the adjacent tissues. The presence of these ions in sufficient concentrations is thought to provoke acute and chronic inflammatory responses. When combined with insufficient primary fixation and the lack of stability during healing and function, fibrous encapsulation of the offending material is a common sequela ( Fig 1-2a). Subsequently, epithelial migration along the interface between the implant and the fibrous capsule led to development of extended peri-implant pockets, and the chronic infections resulting from these pockets led to exposure of the implant framework and its eventual loss ( Fig 1-2b). In general, these implant systems survived for 5 to 7 years before the infections prompted their removal. The infections were particularly destructive of bone and soft tissue in the maxilla ( Fig 1-3).
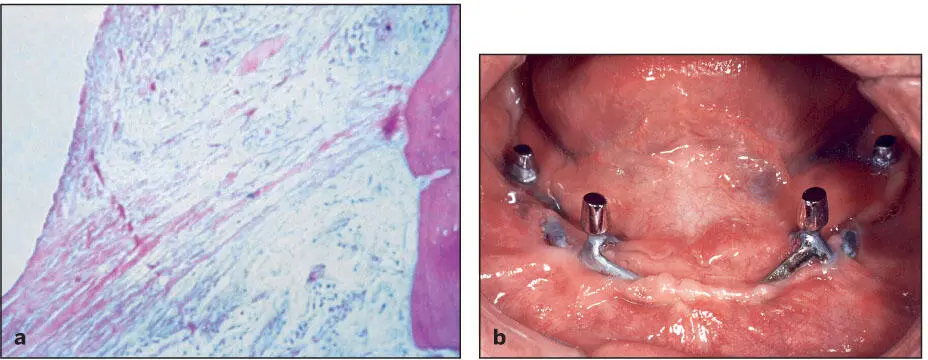
Fig 1-2 (a) Subperiosteal cobalt-chrome implants are enveloped by fibrous connective tissue slings. (Courtesy of Dr R. James.) (b) Epithelial migration led to the formation of extended peri- implant pockets, which in turn developed into chronic infections. The infections led to exposure of the implant struts and eventually loss of the implant.
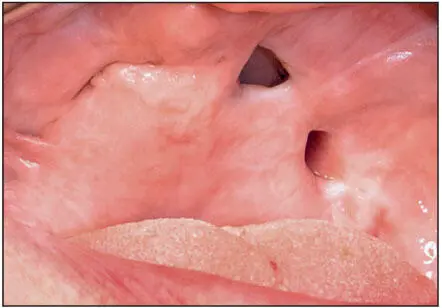
Fig 1-3 Substantial portions of the hard palate were lost secondary to infections associated with a subperiosteal implant. (Courtesy of Dr J. Jayanetti.)
Titanium, however, spontaneously forms a coating of titanium dioxide (TiO 2), which is stable and biologically inert and promotes the deposition of a mineralized bone matrix on its surface. In addition, it is easily machined into precision geometries, and the oxide passivation layer provides corrosion resistance under most oral conditions. Following placement of the implant, a blood clot forms between the surface of the implant and the walls of the osteotomy site. 4Plasma proteins are attracted to the area, accompanied by platelet activation and the release of cytokines and growth factors. 5– 7Some of these signaling molecules induce angiogenesis, and others orchestrate the cascade of wound healing response, which includes the recruitment of local stem cells. These and other repair cells migrate via the fibrin scaffold within the osteotomy site toward the implant surface. The stem cells differentiate into osteoblasts and begin to deposit bone on the surface of the implant and the walls of the osteotomy site, eventually leading to anchorage of the implant in bone (the result of contact and distance osteogenesis 8; see Fig 1-1). The initial events of this process take anywhere from 8 weeks to 4 months depending on the biologic microenvironment and the osteoconductivity (the recruitment of osteogenic cells and their migration to the surface of the implant) of the implant surface.
Читать дальше