Library of Congress Cataloging-in-Publication Data
Names: West, Anthony R., author. | John Wiley & Sons, publisher.
Title: Solid state chemistry and its applications / Anthony R. West, Department of Materials Science and Engineering, University of Sheffield, UK.
Description: Second edition. | Hoboken, NJ : Wiley, 2022. | Includes bibliographical references and index.
Identifiers: LCCN 2021028892 (print) | LCCN 2021028893 (ebook) | ISBN 9781118447444 (hardback) | ISBN 9781118695616 (adobe pdf) | ISBN 9781118695579 (epub)
Subjects: LCSH: Solid state chemistry.
Classification: LCC QD478 .W47 2022 (print) | LCC QD478 (ebook) | DDC 541/.0421–dc23
LC record available at https://lccn.loc.gov/2021028892LC ebook record available at https://lccn.loc.gov/2021028893
Cover Design: Wiley
Cover Image: Courtesy of Anthony R. West
For Sheena, Isla, Graeme, Jenny, Susie and Fraser
Nearly 40 years have passed since the First Edition of this book was published. In the intervening years, two editions of a slimmed down, student version, Basic Solid State Chemistry were published, followed by the Student Edition of what has ultimately become this, the Second Edition. And, several Nobel prizes have been awarded for Solid State Chemistry‐related discoveries! Although several changes to chapter titles and organisation of the main topics have been made, this Second Edition aims still to cover the fundamental science behind the synthesis, structures including defect structures, characterisation, properties and applications of inorganic materials. Relevant aspects of chemistry, physics and materials science are drawn upon to provide a coherent overview of Solid State Chemistry. In the First Edition, an introductory chapter was entitled ‘What is Solid State Chemistry’. With the passage of time, that is felt to be no longer necessary, but instead, is replaced by ‘Solid state chemistry: an overview of the discipline’ which shows the overlap with, and evolution of, emerging areas of Materials Chemistry and Nanomaterials. Organic materials are not excluded, but do not feature strongly here, nor do inorganic‐organic composite or hybrid materials; these are better covered in works on Materials Chemistry.
The major additions to this second edition are many and include: a more extensive overview of the crystal structures of important families of inorganic solids including spinels, perovskites, tungsten bronzes, garnets, pyrochlores and many more; an easy to use, bespoke CrystalMaker® software, accompanied by more than 100 crystal structure models, that can be downloaded free and used to examine these structures on one’s own computer; new methods to synthesise inorganic solids, including sol‐gel methods and thin film deposition techniques such as PLD, MBE, spray pyrolysis, as well as CVD for fabrication of diamond films and amorphous silicon; newer techniques of electron microscopy including EPMA, EELS, Auger, CL and HAADF/Z contrast STEM; major advances in electrical properties of materials, including cuprate superconductors (Nobel prize, 1987), graphene (Nobel prize, 2010), fibre optic communications (Nobel prize, 2009), neutron diffraction and spectroscopy (Nobel prize, 1994), NMR spectroscopy (Nobel prize, 1991), electron microscopy (Nobel prize, 1986), fullerenes (Nobel prize, 1996), polyacetylene (Nobel prize, 2000), lithium batteries (Nobel prize, 2018) and solid oxide fuel cells; novel developments in optical properties, including semiconductor lasers and blue LEDs (Nobel prize, 2014), fibre optics, solar cells and transparent conducting oxides; advances in magnetic properties including giant and colossal magnetoresistance (Nobel prize, 2007), quasicrystals (Nobel prize, 2011) and multiferroic materials; homogeneous and heterogeneous ceramics together with an overview of the new impedance spectroscopy technique and its applications; thermoelectric materials and their applications; multifunctional materials, including MXenes, graphene, other 2D layered structures, TiO 2nanomaterials and Ca 12Al 14O 33superconducting electride. Coverage of the traditional structural materials of glass, cement and concrete, refractories and high temperature ceramics is expanded to include new developments in fluoride glasses for fibre optics, bioglass for tissue engineering applications, geopolymers and novel cements.
Anthony R. West
Sheffield
February 2022
This textbook is supported by a website which contains a variety of supplementary resources:
www.wiley.com/go/west/solidstatechemistry2
Online you will find PowerPoint slides of all figures from the book, as well as solutions to the set of questions. The website also gives you access to a CrystalMaker ®viewer program. The CrystalViewer software is available for Windows and Mac, and is accompanied by a broad array of crystal structures for you to view and manipulate.
CrystalViewer is a visualisation program for displaying and manipulating crystal structures. The CrystalViewer software facilitates the exploration of crystal structures from the book in three dimensions, allowing users to view the structures in different orientations, and highlight/hide different structural features so as to aid the interpretation of complex crystal structures. The CrystalViewer program is accompanied by over 100 crystal structure files; many of these structures relate directly to illustrations from the book, identified by their figure numbers, and a variety of additional structures are provided to complement the concepts and applications discussed in the text.
The CrystalViewer software and accompanying structure files can be downloaded from the companion website at http://www.wiley.com/go/west/solidstatechemistrystudent.
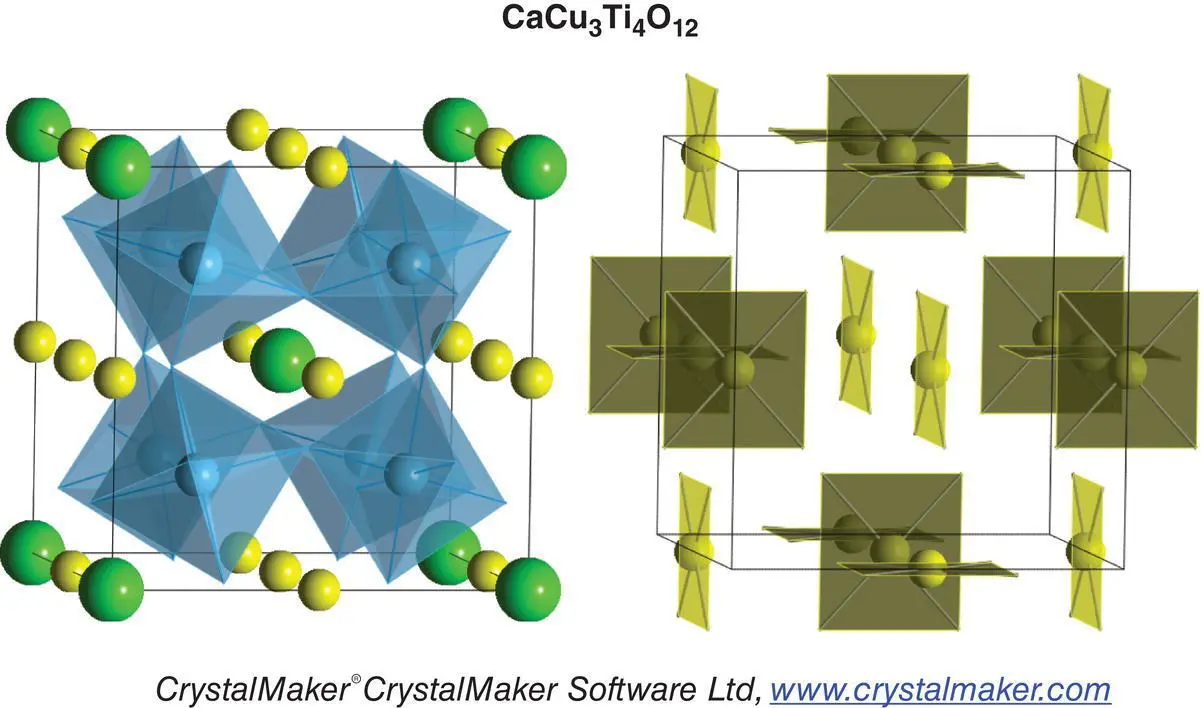
An example of how a crystal structure can appear very different, depending on which aspects are emphasised, is shown here for CaCu 3Ti 4O 12, in which the two diagrams highlight either the TiO 6octahedra or the CuO 4square planar units.
Crystal Structure Library
A Crystal Structure Library is available on the companion website containing >100 structures which can be examined in detail using the CrystalViewer Software. The structures which correspond directly to figures in the book are listed below, with the relevant figure number noted in parentheses. Many more crystal structures are available online, including minerals and other inorganic structures. Further structures may be added from time to time.
Major Inorganic Structure Types (and relevant book diagrams)
| β ‐alumina, NaAl 11O 17(8.23 and 8.24) Anatase BaTiO 3(8.40) bcc metal (2.12) Beryl Bi 4V 2O 11Bixbyite Brass, ZnCu (2.11) Brookite Brownmillerite, Ca 2(Fe,Al) 2O 5(1.42) Brucite CaC 2(1.10) CaCu 3Ti 4O 12(1.42) Calcite Ca silicates (several) CdCl 2(1.40) Cdl 2(1.39) Chevrel Phase, BaMo 6S 8(8.6) Corundum, α ‐Al 2O 3(1.46) CsCl (1.36) Delafossite Diamond (1.33) Eucryptite fcc metal (1.20) Feldspar Fluorite/antifluorite, CaF 2(1.29, 1.30 and 1.34) Garnet, Y 3Fe 5O 12(1.49) GdFeO 3(1.41) Gehlenite hcp metal (1.21) Hollandite (8.27) Ilmenite, FeTiO 3(1.46) K 2NiF 4(1.50) LaB 6Layered double hydroxides (4.11) |
Li 3N (8.32) Li 2MnO 3LiCoO 2/ α ‐NaFeO 2(8.35) LiNbO 3(1.46) Li 3PO 4Li 3SbO 4Li silicates LiAlO 2Magnetoplumbite (9.14) MgB 2(1.51) Mayenite Nasicon, NaZr 2(PO 4) 3(8.27) Melilite Mullite Muscovite Nickel arsenide, NiAs (1.35) Olivine, LiFePO 4(1.45) PbFCl, matlockite (8.6) PbO (3.14) Perovskite, SrTiO 3(1.41) Pyrochlore (1.48) Rock salt, NaCl (1.2, 1.29 and 1.31) Rutile, TiO 2(1.37) Scheelite SiO 2Sodalite Spinel (1.44) Spodumene Talc Tenorite, CuO Tetragonal tungsten bronze (1.43) Willemite Wurtzite, ZnS (1.35) YBa 2Cu 3O 6(8.8) YBa 2Cu 3O 7(8.8) Zinc blende/sphalerite, ZnS (1.29 and 1.33) Zircon ZrCuSiAs (8.6) |
Читать дальше













