James A. Jahnke - Continuous Emission Monitoring
Здесь есть возможность читать онлайн «James A. Jahnke - Continuous Emission Monitoring» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Continuous Emission Monitoring
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:4 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 80
- 1
- 2
- 3
- 4
- 5
Continuous Emission Monitoring: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Continuous Emission Monitoring»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
The new edition of the only single-volume reference on both the regulatory and technical aspects of U.S. and international continuous emission monitoring (CEM) systems Continuous Emission Monitoring
Continuous Emission Monitoring:
Continuous Emission Monitoring, Third Edition
Continuous Emission Monitoring — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Continuous Emission Monitoring», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
These are all scenarios that may cause discrepancies in dilution system measurements. However, these discrepancies can be corrected (i) empirically or (ii) theoretically. Empirical corrections, based on experimental data from installed systems, have been used successfully in specific applications. Lacking experimental data, theoretical expressions can be used and have also been successful in correcting for the effect of gas density variation on the dilution ratio.
Empirical Corrections.
Empirical correction equations for pressure and temperature have been given by Jahnke and Marshall (1994) as follows:
Pressure Correction Equation.
Pressure changes may be caused by changes in either stack pressure or ambient pressure. It is not uncommon to observe an apparent reduction in pollutant gas concentrations when a weather front passes a CEM installation having a dilution system uncorrected for pressure. In one study, this pressure effect was noted as causing a 1% error for each 3.45 in. of water pressure change (Jahnke and Marshall 1994; see Figure 3‐26).
An empirical correction equation that has been used to account for pressure changes in the EPM dilution probe is given in Equation 3‐4:
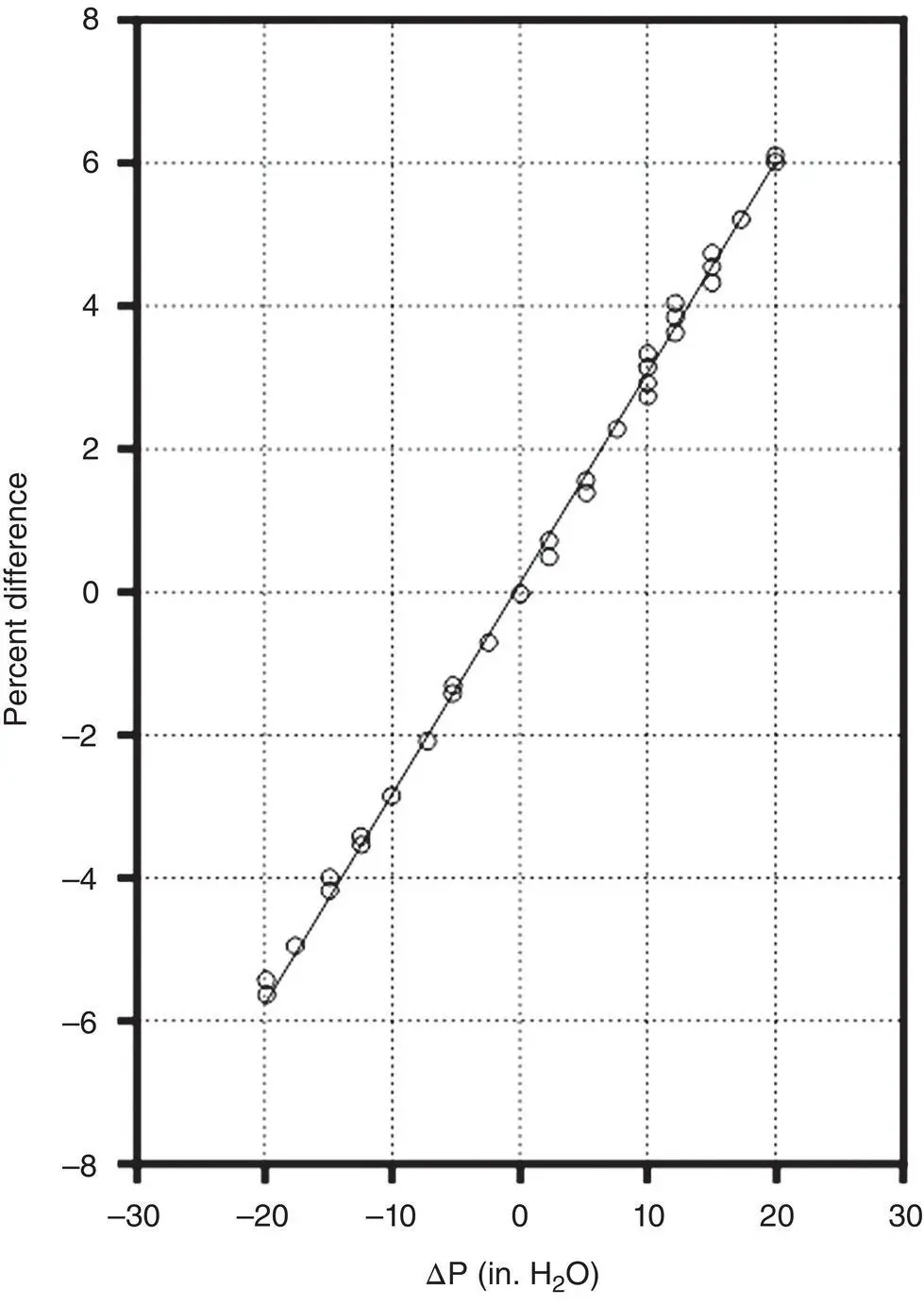
Figure 3‐26 Pressure dependence of the critical orifice dilution system.
Source : Jahnke and Marshall (1994).
(3‐4) 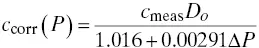
where
∆P is the difference between the stack absolute pressure when the measurement is made and the pressure when the system was calibrated.
This equation appears to be general for dilution systems that do not use mass flow controllers for the dilution air. It would be prudent, however, to check the validity of the empirical coefficients on the installed system and adjust them for any system‐specific characteristics. Procedures for doing this are given in Jahnke and Marshall (1994).
Temperature Correction Equation.
Temperature effects contribute approximately 1% error for each 50 °F change in temperature from the initial dilution probe calibration setting (Jahnke and Marshall 1994). The temperature effect, however, is nonlinear. Temperature fluctuations of this magnitude do not usually occur in most operating units. However, for cycling units and other units that operate infrequently, a common practice has been to perform calibration adjustments to the dilution system when the unit is cold (not operating) and assume that the calibration holds during start‐up and when the unit is hot. This is not a valid assumption and can lead to measurement error greater than 10% due to the nonlinearity of the temperature dependence.
Probe temperature dependence can be minimized by heating the probe, although this has not always been successful during start‐up due to cooling of the heater by initially cool flue gas. A better approach, particularly for cycling units, is to use an external dilution system outside of the stack, where the temperature can be better controlled.
Alternatively, but not as satisfactory as maintaining probe temperature, empirical temperature correction equations can be developed. One equation developed for the EPM dilution probe is given in Equation 3‐5.
(3‐5) 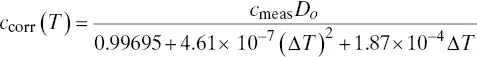
This correction equation appears to be system specific (Jahnke and Marshall 1994) and should only be used as a first approximation for small changes in temperature (e.g. ± 50 °F). It is recommended that the user check the expression for his or her own installation and adjust it as appropriate. It does not appear to work well in cycling units where large swings in temperature are frequent.
Molecular Weight Correction Equation.
Since the flow through the critical orifice is dependent on gas density, molecular weight of the sample gas also affects the dilution ratio. Fluctuation of flue gas moisture and CO 2concentrations can cause errors in the dilution measurements since their concentrations are respectively low (18) and high (44) relative to the usual combustion gas molecular weight of ~30. Also, with regard to earlier Scenario 4, the use of multi‐blend calibration gases containing CO 2initially caused considerable confusion in conducting calibration error (drift) and linearity tests. The higher molecular weight of CO 2increases the density and will cause lower readings if the dilution system was previously calibrated with a cylinder gas containing nitrogen (MW 28) only, as a background gas. Errors due to fluctuations in molecular weight have been calculated to approach up to 7%, depending upon the initial calibration conditions and measurement conditions (McGowan 1994).
This issue was first resolved by Miller (1994) who calculated relative sonic velocities for a variety of multicomponent gas mixtures. A molecular weight correction expression was developed by Appel (1994) for changes in flue gas composition valid under the condition that the dilution system is calibrated initially with single‐blend, nitrogen background cylinder gases:
(3‐6) 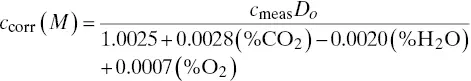
Combined Corrections.
If pressure, temperature, and molecular weight changes are affecting the dilution system response, the equations given above can be cascaded to obtain the following:
(3‐7) 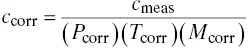
Such an expression may not be necessary for all applications, but it does indicate that some insight must be put into the application and that more than one correction may be necessary.
Theoretical Corrections.
Although the theory of the critical orifice has been examined in detail, the construction and operation of dilution probe and its analogues may not satisfy all the underlying assumptions of the theory. Theoretical aspects of dilution systems were studied by Munukutla (1992) and Jahnke and Marshall (1994), Romero and Associates (1999), and Batug and Associates (2004). The extended formulation of Jahnke is provided here.
Dilution system theory begins from the theoretical equation for critical flow (see ASME 1971; Green and Perry 2007; Sadegh and Worek 2017; Shapiro 1953). This equation is used to obtain a corrected dilution ratio, D , from a new set of gas conditions that differ from the conditions under which the system was originally calibrated for the initial dilution ratio, D o(Jahnke and Marshall 1994; Munukutla 1992):
(3‐8) 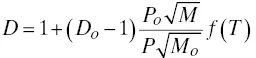
Instead of using Equation 3‐3( c = c meas D o) to calculate the source‐level concentration from the measured analyzer data, the following equation can be used to correct the data to the actual conditions of measurement:
Читать дальшеИнтервал:
Закладка:
Похожие книги на «Continuous Emission Monitoring»
Представляем Вашему вниманию похожие книги на «Continuous Emission Monitoring» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Continuous Emission Monitoring» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.












