Nitric Oxide in Plants
Здесь есть возможность читать онлайн «Nitric Oxide in Plants» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Nitric Oxide in Plants
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:5 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 100
- 1
- 2
- 3
- 4
- 5
Nitric Oxide in Plants: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Nitric Oxide in Plants»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
Examines the beneficial roles of nitric oxide in growth and stress tolerance regulation through its involvement in tolerance mechanisms Nitric Oxide in Plants: A Molecule with Dual Roles
Nitric Oxide in Plants: A Molecule with Dual Roles
Nitric Oxide in Plants — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Nitric Oxide in Plants», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
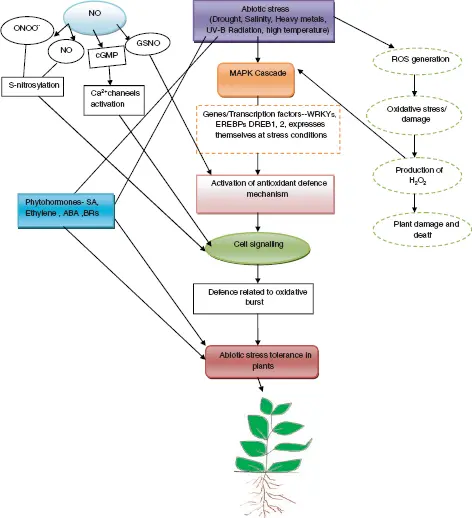
Figure 1.3 Schematic illustration of NO signaling in abiotic stress.
Mackerness et al. (2001) demonstrated the involvement of NO in plant response to UV-B radiation, demonstrating poststress induction of chalcone synthase expression, a rise in NOS-type protein activity, and an increase in NO levels. According to the findings of Shi et al. (2005), NO positively shields plants against UV-B radiation, most likely through increased activity of the antioxidative system. NO-donor treatment of potato tubers prior to UV-B irradiation resulted in the development of approximately 50% more healthy leaves than plants not subjected to NO treatment (Neill et al. 2003). Exogenous NO has been shown to reduce the deleterious effects of heavy metals, ethylene, and herbicides on plants in response to alternative abiotic stresses (Kopyra and Gwóźdź 2003). The authors explained the protective effect as a result of NO-donor treatment of plant materials by the effect of NO on the elevation of activity of antioxidative enzymes, particularly SOD (Kopyra and Gwóźdź 2003).
According to the cited authors, such a chain of events could effectively reduce the amount of ROS produced during stress, thereby limiting oxidative stress in plant cells. Similarly to salinity stress, NO-donor treatment of rice seedlings resulted in loss minimization (Uhida et al. 2002). When NO was used, plant growth was increased, along with the maintenance of acceptable photosystem II activity, an increase in antioxidative protein activity, and thus the expression of specific salinity stress resistance genes. On the other hand, prolonged stress situations may result in the production of NO and NO-derived products, resulting in specific responses, referred to as nitrosative stress. Valderrama et al. (2007) found that salinity stress elicited the assembly of RNS, i.e. NO, GSNO, and RSNO, as well as an increase in tyrosine-nitrated proteins, which are sensitive markers of nitrosative stress, in olive leaves. Furthermore, they demonstrated that vascular tissues may play an important role in the distribution of NO-derived forms during nitrosative stress and in signaling processes. Tissue damage, which is usually due to a microorganism invading a cell, frequently results in NO production and H 2O 2accumulation (Delledonne et al. 1998). According to Orozco-Cardenas and Ryan (2002), injury does not induce NO synthesis; however, the use of exogenous NO inhibits the method of NO generation and expression of wound-inducing genes.
It is important to note that many types of abiotic stress (cold and heat stress, salt and drought stress) increase polyamine (PA) synthesis (Bouchereau et al. 1999). Tun et al. (2006) discovered that PAs significantly increase NO generation. Work on Arabidopsis seedlings confirms that NO acts as a channel between PA-mediated stress responses and an alternative stress mediator, with NO as a stepping stone.
By scavenging ROS, NO plays a significant role in inhibitor defense against many abiotic stresses. Salinity stress has a negative impact on plant morphological traits and diffusion balance. Additionally, it promotes membrane disintegration, DNA damage, particle discharge, and death. NO has been shown to have a potential effect on diffusion stress tolerance as well as increased spermatophyte growth in rice, lupin, and cucumber when subjected to salt stress (Uchida et al. 2002; Kopyra and Gwóźdź 2003; Fan et al. 2007, 2013; Barakat et al. 2012). Similar evidence has been reported demonstrating the potential effect of NO in mitigating salinity stress in alfalfa, barley, jatropha, chickpea, and sunflower (Nabi et al. 2019). In Oryza sativa , a NO donor SNP inhibited accumulation, reduced ROS generation, and improved root growth (Kushwaha et al. 2019). In various plant species, the potential role of gas in mitigating serious metal toxicity has been investigated (Ahmad et al. 2018; Yuanjie et al. 2019; Bhuyan et al. 2020; Wei et al. 2020; Khator et al. 2021). As a result, it is clear that NO gas could be a potential mitigating agent for abiotic stresses.
1.4.1 Crosstalk of Nitric Oxide with Other Phytohormones in Plants to Confer Abiotic Stress Tolerance
NO is involved in advanced signal mechanisms, as well as synergistic collaboration with phytohormones and alternative secondary signal molecules, to confer stress tolerance in plants. NO has been linked to a variety of phytohormones, including gibberellins, brassinosteroids, and ABA. The interaction of NO with phytohormones has been studied in a variety of ways. NO and plant hormones work together to regulate a wide range of physiological responses in plants. By activating Ca 2+and calcium‐dependent protein kinase via downstream signals, NO and auxin promote root development (Pagnussat et al. 2002). Similarly, the interaction of NO and auxin promotes Cd tolerance in the rosid dicot genus Truncatula by reducing auxin degradation (Xu et al. 2010). Furthermore, there is a growing of evidence pointing to the effect of NO in reducing serious metal toxicity (He et al. 2012; Yuan and Huang 2016; Wei et al. 2020). Iron deficiency, on the other hand, stimulates the assembly of auxin and increases NO levels, thereby upregulating ferric-chelate enzyme activity in Arabidopsis .
Various studies have emphasized the role of cytokinins in plant organic process processes, cellular division, and leaf senescence. Cytokinins and NO interact in a variety of ways, including synergistic and antagonistic responses. NO and cytokinins interact synergistically in response to drought stress causing leaf senescence, cellular division, and photosynthetic activity (Mishina et al. 2007; Shao et al. 2010; Shen et al. 2013). NO signaling via gibberellins induces multiple physiological responses in plants, including seed germination, root growth (Lozano-Juste and León 2011; Sanz et al. 2015), photosynthetic activity, and nutrient use potency. NO and gibberellins also have an antagonistic relationship because NO suppresses gibberellic acid signal events and signal transduction by promoting the accumulation of DELLA proteins (Asgher et al. 2017). Wu et al. (2014) discovered that gibberellins work antagonistically with NO to manage root growth in Arabidopsis at low and high P concentrations. Likewise, NO production is required for ABA-induced stomatal closure in guard cells (Neill et al. 2002). The role of NO–ABA interactions in drought stress and UV-B radiation stress has been well established in controlling stomatal closure and inhibitor defense machinery (Neill et al. 2008; Tossi et al. 2009).
Several studies have shown that ABA and NO interact in plant physiological responses and signaling mechanisms (Castillo et al. 2015; Asgher et al. 2017; Wang et al. 2020). Furthermore, NO and ethylene have an antagonistic interaction because NO inhibits ethylene synthesis and action by inhibiting leaf senescence and ripening (Leshem et al. 1998; Manjunatha et al. 2010). However, serious physiological responses to NO interactions with ethylene are being studied in Arabidopsis , Cucumis , and Nicotiana (Ederli et al. 2006). As a result, it is clear that the interaction of NO with phytohormones in abiotic stress tolerance is supported by a variety of evidence. Consider the interaction of NO with various hormones such as brassinosteroids, jasmonates, and polyamines (Liu et al. 2014; Lau et al. 2021; Nahar et al. 2016). The interaction of NO with hormones activates the advanced signal cascade, inducing a variety of responses to environmental stresses.
Despite the fact that there is conflicting evidence regarding the interference mechanism of NO with hormones, future research on the mechanisms of interaction of multiple hormones with NO and their potential pathways is required to be addressed in terms of stress responses and plant growth traits ( Table 1.1).
Читать дальшеИнтервал:
Закладка:
Похожие книги на «Nitric Oxide in Plants»
Представляем Вашему вниманию похожие книги на «Nitric Oxide in Plants» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Nitric Oxide in Plants» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.












