Perovskite Materials for Energy and Environmental Applications
Здесь есть возможность читать онлайн «Perovskite Materials for Energy and Environmental Applications» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Perovskite Materials for Energy and Environmental Applications
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:5 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 100
- 1
- 2
- 3
- 4
- 5
Perovskite Materials for Energy and Environmental Applications: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Perovskite Materials for Energy and Environmental Applications»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
The book provides a state-of-the-art summary and discussion about the recent progress in the development and engineering of perovskite solar cells materials along with the future directions it might take.
Audience
Perovskite Materials for Energy and Environmental Applications — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Perovskite Materials for Energy and Environmental Applications», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
Dye-sensitized solar cell (DSSC) is the first third-generation solar cell and perovskite solar cell (PSC) has evolved from it, as shown in Figure 2.2. The DSSC consists of layers of glass/FTO (fluorine-doped tin oxide)/mesoporous layer of titanium dioxide/light-absorbing dye/electrolyte/FTO/ glass. Despite the impressive research work, DSSC was able to achieve max 13% PCE [2]. The major advantages of DSSC include low fabrication costs and a simple manufacturing process, although disadvantages include the absorption layer should be more than 10 µm to ensure complete light absorption and the phenomenon of light bleaching occurs due to organic dyes [1]. Solid state DSSC (SSDSSC) contains solid hole conductors instead of liquid electrolytes and the thickness is reduced to 2 µm. The hole conductor contains wide bandgap material, such as Spiro-OMeTAD or PEDOT or P3HT. In 2009, Kojima et al. [3] utilized organolead halide as a sensitizer to achieve the power conversion efficiency (PCE) of 3.8%. In 2012, for the first time, an all-solid-state PSC was prepared and achieved a PCE of 9.7% [4]. This showed the potential of PSC toward future cost-effective and high-performance solar cells. A considerable amount of research has been done on PSC in a short time. The highest efficiency achieved was 22.1% in 2016 [5]. For commercialization, stability and toxicity of the PSC should be looked upon.
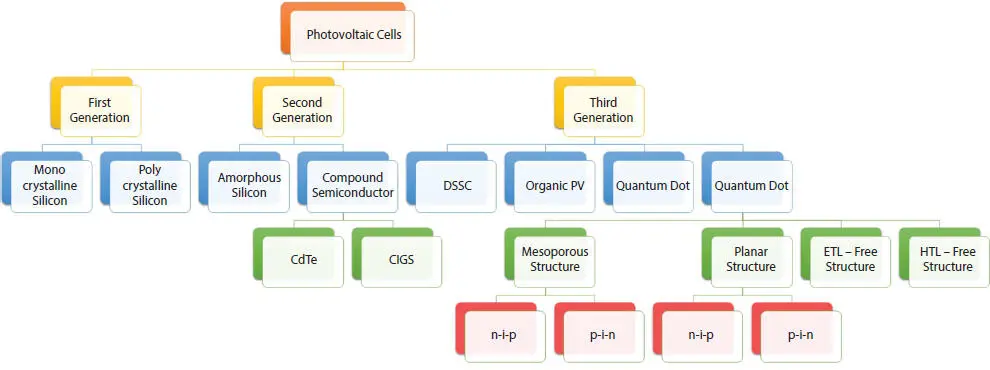
Figure 2.1 Generations of the photovoltaic cells.
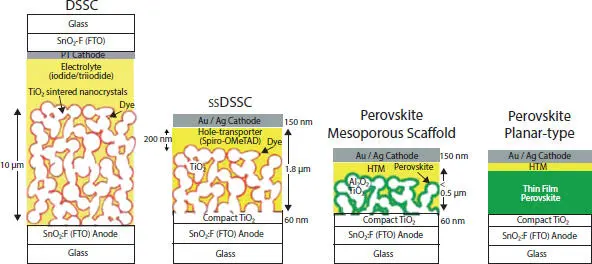
Figure 2.2 Evolution of perovskite solar cell from reduction of size of light absorbing layer from 10 µm (DSSC) to less than 0.5 µm (perovskite solar cell). Reprinted with permission. Copyright {2013} American Chemical Society [1].
2.2 Structure
Perovskite is the material having the same compound formula as CaTiO 3. Perovskite materials have the general formula of ABX 3. Gustav Rose discovered this compound from the Ural Mountains of Russia in 1839 and was named after the mineralogist Lev Perovskite (1792-1856). The Perovskite structure was first described by Victor Goldschmidt in 1926. Perovskite can be divided into two types—inorganic oxide perovskite and halide perovskite. Halide perovskite are further divided into alkali halide perovskite and organo-metal halide perovskite where in A, B, and X vary as mentioned in Table 2.1[6].
Table 2.1 Types of perovskite structures.
| Types of perovskite structure | A | B | X |
| Inorganic oxide perovskite | Divalent ions—Pb 2+, Sn 2+, Ca 2+, Mg 2+, etc | Tetravalent metal ions— Ti 4+, Sn 4+, etc | O |
| Alkali halide perovskite | Monovalent ions— Na +, Li +, etc | Divalent ions— Pb 2+, Sn 2+, Ca 2+, Mg 2+, etc | Halogen—Cl −, Br −, I − |
| Organo-metal halide perovskite | Organic cations— methylammonium (CH 3NH 3) and formamidinium (HC(NH 2) 2) | Divalent ions—Pb 2+, Sn 2+, Ca 2+, Mg 2+, etc | Halogen—Cl −, Br −, I − |
Perovskite has a cubic structure as shown in Figure 2.3. A is situated on the vertex, B is at the center, whereas X is situated at the face center. It is equivalent to an octahedral structure.
A small defect in the lattice decreases the symmetry of a crystal [7]. The perovskite mineral is itself distorted, and because of this distortion, many physical properties, like electronic, magnetic, and dielectric behavior, are impacted. The ideal cubic perovskite structure is not common. It has been reported that if the grain surface of perovskite (CH 3NH 3PbI 3) is chemically modified by spin-coating of 4-ABPACl, butylphosphonic acid 4-ammonium chloride then the efficiency and stability both improve. 4-ABPACl forms crosslink with perovskite by the formation of hydrogen bond between perovskite (CH 3NH 3PbI 3) iodine and –PO(OH) 2, –NH 3 +, as a result, the cohesion force increases between neighboring perovskite grains [8].
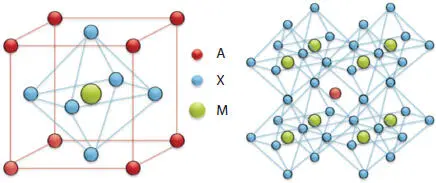
Figure 2.3 The crystal structure of the perovskite [6].
2.3 Working Mechanism of PSC
The working principle of the PSC can be understood by the working of the DSSC and organic photovoltaic. In PSC, the perovskite is used as a light sensitizer, the same as dye in DSSC. The three functions performed by PSC are formation of the exciton (electron-hole pair) by the adsorption of the photon, charge transport, and charge extraction. When light falls on the perovskite, excitons are formed. Because of the difference in the binding energy of exciton of perovskite material, free carriers (free electron and hole) are formed from exciton, which can either generate current or can recombine. Because of the low recombination rate of free carriers in perovskite material and high charge mobility, the diffusion distance and lifetime of the carrier are long. And as a result, PSC is able to achieve such a high efficiency. Highly crystalline CH 3NH 3PbI 3perovskite material is able to achieve charge carrier diffusion length as long as 10 micrometers [9]. As shown in Figure 2.4, the electron is generated in perovskite and then moves to the electron transporting layer (ETL) and finally to the anode. Simultaneously, holes generated in perovskite, move to the hole transporting layer (HTL) and finally to the cathode. The electron migrates to the anode which can be FTO or ITO. The hole migrates to the cathode, which is either Ag or Au [10].
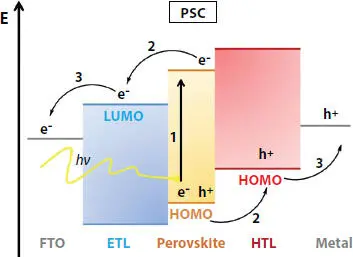
Figure 2.4 Working of the perovskite solar cell [11].
2.4 Device Architecture
Device arrangement is definitely one of the most significant aspects for the performance of the PSC. Depending on the position of the transport layer in the exterior portion, PSC can be classified into two configurations like mesoporous structure and planar structure as depicted in Figure 2.5. These two configurations are further classified into regular (n-i-p) and inverted (p-i-n) as shown in Figure 2.6.
Other two configurations are also possible like ETM-free structure and HTM-free structure, where P-contact is ETM, whereas N-contact is HTM. The brief overview of mesoporous structure and planar structure is discussed further in the chapter.
2.4.1 Mesoporous Structure
A typical n-i-p mesoporous structure is shown in Figure 2.6.a, ETM is on the exterior portion. The first arrangement of the PSC to be tested was a conventional n-i-p mesoporous structure in which the light-harvesting dye was replaced by the lead halide perovskite materials (CH 3NH 3PbBr 3and CH 3NH 3PbI 3) in a traditional DSSC [3]. Mesoporous structure of PSC is widely used because of its high porosity and high surface area. As shown in Figure 2.6.a, the mesoporous n-i-p PSC consists of FTO, ETM, a mesoporous layer of metal oxide, perovskite layer, HTM, and an electrode layer. In mesoporous p-i-n PSC, the HTM layer is on the exterior portion as shown in Figure 2.6.b. The perovskite material adheres to the mesoporous structure to increase the light-absorbing area of perovskite, which results in increased efficiency. The mesoporous structure results in efficient crystallization of perovskite and electron injection [9]. There is a depletion region at the TiO 2side of the TiO 2/MAPbI 3interface, which provides contribution in separation of photogenerated charge carriers and hence it results in high performance of TiO 2-based cells [14]. TiO 2mesoporous layer helps to transport the electrons, block holes, and inhibit the recombination of the electron and hole pair. The electron diffusion length and recombination lifetime is governed by the mesoporous layer of TiO 2and hence is not varied by perovskite composition [15].
Читать дальшеИнтервал:
Закладка:
Похожие книги на «Perovskite Materials for Energy and Environmental Applications»
Представляем Вашему вниманию похожие книги на «Perovskite Materials for Energy and Environmental Applications» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Perovskite Materials for Energy and Environmental Applications» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.












