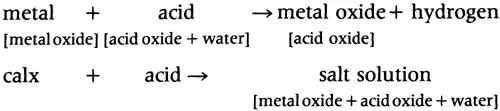
Whatever the merits of the claim that Lavoisier was the first to grasp that water was a compound of hydrogen (meaning ‘water producer’) and oxygen, the important point was that he could now explain why metals dissolved in acids to produce hydrogen. This, he asserted, came not from the metal (as the phlogistonists claimed, some even identifying phlogiston with inflammable air), but from the water in which the acid oxide was dissolved:
Although it was left to Davy and others to develop the point, the understanding of water also helped lead to a hydrogen theory of acidity.
Lavoisier was now in a position to bring about a revolution in chemistry by ridding it of phlogiston and by introducing a new theory of composition. His first move in this direction was made in 1785 in an essay attacking the concept of phlogiston. Since all chemical phenomena were explicable without its aid, it seemed highly improbable that the substance existed. He concluded:
All these reflections confirm what I have advanced, what I set out to prove [in 1773] and what I am going to repeat again. Chemists have made phlogiston a vague principle, which is not strictly defined and which consequently fits all the explanations demanded of it. Sometimes it has weight, sometimes it has not; sometimes it is free fire, sometimes it is fire combined with an earth; sometimes it passes through the pores of vessels, sometimes they are impenetrable to it. It explains at once causticity and non-causticity, transparency and opacity, colour and the absence of colours. It is a veritable Proteus that changes its form every instant!
By collaborating with younger assistants, whom he gradually converted to his way of interpreting combustion, acidity, respiration and other chemical phenomena, and by twice-weekly soirées at his home for visiting scientists where demonstrations and discussions could be held, Lavoisier gradually won over a devoted group of anti-phlogistonists. Finding that editorial control of the monthly Journal de physique had been seized by a phlogistonist, Lavoisier and his young disciple, Pierre Adet (1763–1834), founded their own journal, the Annales de Chimie in April 1789. The editorial board soon included most converts to the new system: Guyton, Berthollet, Fourcroy, G. Monge, A. Seguin and N. L. Vauquelin. This is still a leading chemical periodical. While Director of the Academy of Sciences from 1785, Lavoisier was also able to alter its structure so that the chemistry section consisted only of anti-phlogistonists.
It is significant that Lavoisier’s new theory was one of acidity as much as combustion. Stahlian chemists had not foreseen that there were many types of ‘airs’ or gases, but, as Priestley’s career shows, they actually had little difficulty in conceptualizing them within a phlogistic framework. The appearance of gases also led to a modification in the phlogistic theory of acidity. According to Stahl, vitriolic acid (sulphuric acid) was the universal acid – ‘universal’ in the sense of being the acid principle present in all substances that displayed acidic properties. However, with the discovery of fixed air, several chemists, led by Bergman in Sweden, had decided that this, not vitriol, was the true universal acid. Such a view was argued vociferously by the Italian, Marsilio Landriani, during the 1770s and 1780s. Landriani claimed to have found evidence that fixed air was a component of all three mineral acids as well as the growing number of vegetable acids such as formic, acetic, tartaric and saccharic acids. It was really this theory of acidity that Lavoisier had to challenge in the 1780s.
Lavoisier’s method was to challenge the theory as displayed in the French translation undertaken by his wife of Richard Kirwan’s Essay on Phlogiston and the Constitution of Acids. He was able to convince Kirwan that the acidity of fixed air was sufficiently explained by the fact that it contained oxygen. The irony here was that Lavoisier’s new theory retained in effect the Stahlian notion of a universal acid principle in the form of oxygen. In practice, the explanation of properties by principles was not to last much longer after the advent of Dalton’s atomism and the evidence that not all acids contained oxygen.
The demonstration by Hales that fixed air formed part of the composition of many solids and liquids had also given rise to speculations that this air was vital to vegetable and animal metabolisms. For example, in 1764, an Irish physician, David Macbride, concluded that ‘this air, extensively united with every part of our body’, served to prevent putrefaction, a prime example of which was the disease called scurvy. The recognized value of fresh vegetables in inhibiting scurvy, he suggested, was due to their fermentative powers. The fixed air that they produced during digestion served to prevent putrefaction inside the body.
It was this suggestion that inspired Priestley to investigate the effects of airs on living organisms – a programme of research that was to form the basis of Davy’s earliest research some time later. Initially, in 1772, Priestley concluded that fixed air was fatal to vegetable life, but this was probably due to the fact that he used impure carbon dioxide from a brewery, or that he was using it in excess. Others, including Priestley’s Mancunian friend, Thomas Henry, found the opposite, that flowers thrived in fixed air. It was while repeating these findings that Priestley discovered that, in the presence of sunlight (but not otherwise), plants growing in water, such as sprigs of mint, gave off dephlogisticated air. This had already been anticipated in 1779 by Jan Ingenhousz (1730–99) who, together with Jean Senebier (1742–1809) in Geneva, laid the foundations of a theory of photosynthesis in plants.
Three particularly important converts to the new chemistry were Guyton (whose work had earlier catalysed Lavoisier’s interest in combustion), Claude-Louis Berthollet (1748–1822) and Antoine Fourcroy (1755–1809). Berthollet’s conversion to Lavoisier’s views seems to have arisen because of his own perturbation at the weight changes involved in calcination, to which Guyton had drawn attention. In his Observations sur l’air (1776), Berthollet explained acidity and weight changes in combustion by means of fixed air, and otherwise incorporated Lavoisier’s work on oxygen into the phlogiston framework. It was the analysis of water, together with increasing personal contact with Lavoisier in the Academy, where they found themselves drawing up joint referees’ reports, that converted Berthollet to Lavoisier’s position by 1785. In fact, Berthollet always had certain reservations. In particular, he never accepted the oxygen theory of acidity, and his investigation of chlorine (first prepared by Scheele in 1774 and assumed by Lavoisier to be oxygenated muriatic acid) seemed to confirm his doubts. In later life he also firmly rejected the notion that chemical properties could be explained in terms of property-bearing principles.
Fourcroy was Lavoisier’s principal interpreter to the younger generation. His ten-volume Système des connaissances chimiques (1800) codified and organized chemistry for the next fifty years around the concepts of elements, acids, bases and salts. Fourcroy saw this structure not only as ‘consolidating the pneumatic doctrine’ but as affording ‘incalculable advantage(s)’ for learning and understanding chemistry (see Table 3.1).
While still a phlogistonist, Guyton was much exercised by the inconsistent nomenclature of chemists and pharmacists. Unlike botany and zoology, whose terminology had been revised and made more precise earlier in the century by the
Читать дальше













