● Due to there being no traditional air shower, and the filling tips being completely enclosed within the parison, it is not possible to perform continuous viable and non-viable particle monitoring throughout the batch. Therefore, it is not possible to classify this area as Grade A in the same manner as for shuttling machines. Any particle monitoring device placed within the closed parison may interfere with the velocity and direction of the sterile air. This may cause the parison to collapse onto the filling tips. Once extrusion is stopped at the end of batch, the filling environment ceases to exist. Therefore, any air samples taken of the air leaving the parison head will not provide meaningful data.
Note
It can be argued that the sterile environment in which the filling tips are present during filling is actually the same as within the sealed, filled unit. Demonstrating that the filling environment is of the appropriate quality therefore requires consideration of the entire sterility assurance ‘package’ for the batch/process, i.e. monitoring of critical parameters/alarms during each batch, parison support filter integrity tests at the end of each batch, media fills, extruder validation, bioburden of incoming polymer etc.
● No operator intervention is possible within the sterile environment once the parison is formed.
3.3 Additional Applications
The majority of BFS machines operate as described above, although there are variations on the principle. Certain machines can insert devices before container closure, e.g. syringes, rubber stoppers, and eye droppers. It is important to consider that any device to be inserted during the blow-fill-seal process must be appropriately sterilised and handled to ensure its sterility is maintained.
In addition, some machines also have co-extrusion capabilities, which can provide an additional barrier to oxygen and/or volatiles migration into the final container. It can also provide an additional barrier to reduce water loss out of the final container. A typical co-extrusion machine has 5 extruders (Fig. 3).
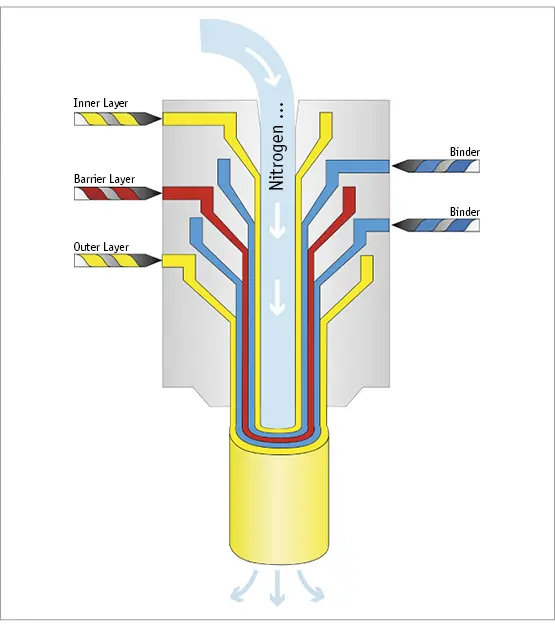
Fig. 3 Schematic representation of the co-extrusion process.
4. ADVANTAGES & CHALLENGES OF BLOW-FILL-SEAL TECHNOLOGY
4.1 Advantages
Blow-Fill-Seal technology offers 2 highly significant advantages over “traditional” aseptic filling operations.
1. In BFS operations, personnel should not normally be present in the filling area during the filling process, thereby removing the greatest potential source of microbial contamination from the operation.
2a. For shuttling machines, the containers are formed immediately before filling, are filled under controlled conditions, and are sealed immediately after filling. Therefore, the exposure time to the environment for any individual product units is only a few seconds.
Конец ознакомительного фрагмента.
Текст предоставлен ООО «ЛитРес».
Прочитайте эту книгу целиком, купив полную легальную версию на ЛитРес.
Безопасно оплатить книгу можно банковской картой Visa, MasterCard, Maestro, со счета мобильного телефона, с платежного терминала, в салоне МТС или Связной, через PayPal, WebMoney, Яндекс.Деньги, QIWI Кошелек, бонусными картами или другим удобным Вам способом.













