BFS technology lacks harmonisation and specific standards on a worldwide basis. As a result, the technology has developed in an isolated fashion, with each company and each regulatory agency establishing its own interpretation of acceptable BFS practice.
In 1989, the Pharmaceutical Blow-Fill-Seal International Operators Association (BFS IOA) was established as an interest group of pharmaceutical and associated companies actively involved with BFS processing. The Association was formed to provide its members with an opportunity to exchange ideas and opinions, and to formulate agreement on operating standards. It also provides a forum to speak with a unified voice to machine manufacturers, commercial suppliers, and regulatory bodies. The Association has expanded worldwide and now has over 60 member companies.
In an attempt to establish a common understanding of acceptable practice in BFS processing, the Association first published a “Points to Consider for Pharmaceutical Blow-Fill-Seal Manufacturing Operations” (PTC) document in September 1993. The document addressed points specific to BFS processing but also covered many more general areas. The current PTC document focuses on issues specific or unique to BFS technology and has undergone periodic review and systematic updates since its inception.
This document is the culmination of several reviews during 2010 and 2011 and the most current version is from March 2012.
The document is structured into 3 main sections covering design, start-up/validation, and routine operation. Within each section, Product, Equipment and Facility are considered.
|
Design |
Initial start-up |
Routine operation |
| Product |
 |
 |
 |
| Equipment |
 |
 |
 |
| Facility |
 |
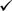 |
 |
2. OBJECTIVE
The objective of the ‘Points to Consider’ document is to provide recommendations specific to the operation of Blow-Fill-Seal technology for the manufacture of sterile pharmaceuticals and liquid medical devices. The principles of BFS technology as applied to filling are considered to be the same in terms of machine process for both aseptically filled and terminally sterilised products.
This document provides information to supplement and to assist with interpretation of international standards and regulatory guidance from the perspective of BFS operations, and considers specific aspects of BFS operation which are not covered by existing published information.
The PTC is intended as a guide for industry and is not meant to supplant or duplicate any existing regulatory guidance. A list of current regulatory guidance references is provided in the Appendix.
3. BFS PROCESS OUTLINE
Blow-Fill-Seal technology is a pharmaceutical filling process in which containers are formed from a thermoplastic granulate, filled with product and then sealed in a continuous, integrated, automatic operation. Originally developed for use in other industries, BFS technology has been adapted for use in the manufacture of sterile pharmaceutical, medical device, biological, and veterinary products. The two most common types of BFS machines are the shuttling machine (with parison cut) and the rotary machine (closed parison) types. Both are considered in this document.
In the process, bulk solution/suspension is delivered to the filling machine, through the filling system to the filling needles (“mandrels”).
Polymer granules are extruded under pressure (up to 350 bar) as a hot (approx. 180°C) mouldable plastic tube (“parison”) or set of parisons.
3.1 Shuttle Type Machines
A downward flow of sterile filtered air is passed through the parison (parison support air) to prevent collapse of the molten tube. The 2 halves of a mould close around the parison(s) to form the body of the container(s). Simultaneously, the newly formed container in the mould is cut free from the parison by a “knife”.
The mould is transferred to the filling position.
The filling mandrel is lowered into the plastic tube and the container is shaped either by vacuum or with sterile blowing air or other inert gas supplied by the mandrel. Subsequently, the container is filled with the required volume of the product. During the liquid fill, headspace gas is displaced from within the container, either through the mandrel or direct to room atmosphere, depending on the container configuration.
The filling mandrel is then raised and the containers are sealed automatically by forming the still hot plastic above the main mould, by closing the upper section of the mould, (head/seal mould). The entire mould assembly then opens, releasing the filled container(s) that have been formed, filled and sealed.
This complete cycle generally takes between 10–20 seconds depending on container design and volume of fill. Parison extrusion continues, as the mould returns to the first station, and the cycle repeats (Fig. 1).
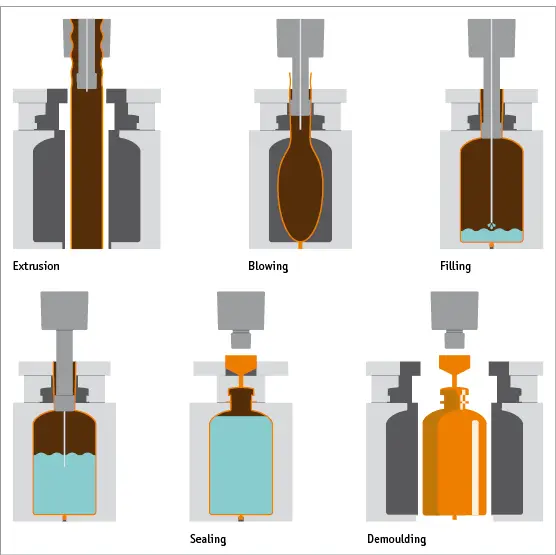
Fig. 1 Schematic representation of the open parison Blow-Fill-Seal process.
3.2 Rotary Filling Machines
There are a number of significant differences between shuttling zone machines and rotary filling machines:
● Rather than 1 or 2 sets of moulds, there are typically 15 sets located on 2 rotating chains.
● The units are produced in 1 continuous ribbon. This means that there is no ‘shuttling zone’ or knife cut required for machines of this type. As a result, particle generation is generally reduced and the units are not open to the environment at any point during the process (Fig. 2a and 2b).
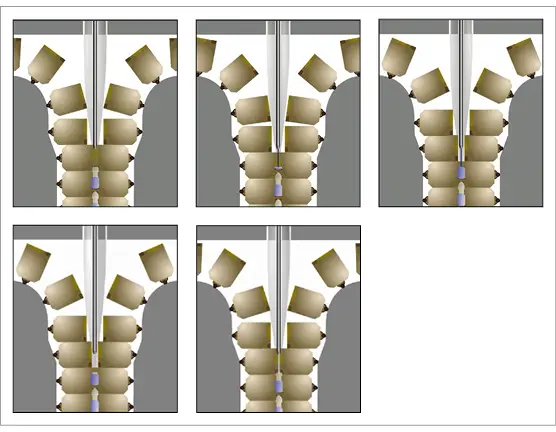
Fig. 2a Schematic representation of the closed parison Blow-Fill-Seal process.
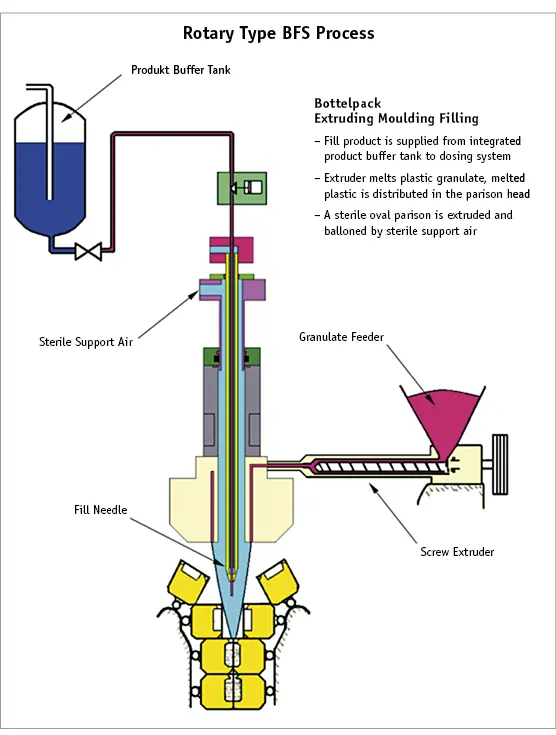
Fig. 2b Schematic representation of the closed parison Blow-Fill-Seal process.
● There is no traditional air shower as seen on ‘open parison’ machines. When not filling, the filling tips are retracted and reside completely within the extruder head and are flushed with sterile air. During filling, the filling tips are completely enclosed by the continuously extruding parison, which is sealed at the bottom by the continuous formation of the units. Throughout the whole time, the tips are surrounded by sterile air. Any loss of pressure inside the parison or not enough air entering into the parison will lead to a too small parison width. This will lead to filling being suspended and the needles will retract into the parison head. The sterility of the tips can therefore be maintained.
Читать дальше