High affinity is important, but it is not sufficient to ensure that an antibody bound to an epitope will retain its hold on the epitope. Because the binding between antigen-binding sites and epitopes is noncovalent, the interaction is reversible. Thus, there is an off-rate, as well as an on-rate, associated with antibody binding to an epitope. The importance of valence is that an antibody with a higher valence will be significantly less likely to detach from the antigen to which it is bound. If two antigen-binding sites of an antibody monomer bind to two adjacent epitopes on an antigen, the probability that both of them will detach at the same time is much lower than the probability that a single antigen-binding site will detach from its epitope. Thus, higher valence can improve the apparent strength of binding of an antibody to an epitope by orders of magnitude.
The differences in affinity and avidity between the first responders IgM/IgA and the adaptive IgG antibodies become important when one considers the roles that these different antibodies play in the immune response. Antibodies, such as IgM and sIgA, that appear early and have lower affinity and less specificity for the invading microbe need to have a higher valence number so that they can increase the avidity for the foreign antigen by binding more epitopes, up to 4 for sIgA or 10 for IgM. High avidity for an antigen is more effective at neutralizing microbes and toxins, blocking their binding and entry into cells. For IgM, high avidity significantly enhances the activation of complement and clustering of receptors to stimulate immune cells. Moreover, high avidity leads to agglutination that enhances opsonization of the microbes by phagocytic cells and clearance of the microbe from the circulation. Rapid clearance is a desirable feature because the longer antibody-antigen complexes remain in circulation, the more likely they are to deposit in the kidneys or other blood-filtering organs, where the complexes can activate complement and cause an inappropriate inflammatory response that damages the organ.
In contrast, IgG is a monomer and can bind only two epitopes, but because it is the product of an adaptive immune response, its low valency and hence low avidity is countered by its much higher affinity and specificity for the foreign antigen. Circulating IgG antibodies can not only bind very tightly to neutralize a specific microbe or toxin, but also mediate opsonization of the specific microbe through binding to Fc receptors on phagocytes. Antigen-bound IgG can also mediate complement activation through binding to a C1 complex.
Cytotoxic T Cells, Also Known as Cytotoxic T Lymphocytes (CTLs)
Cytotoxic T Lymphocytes: Critical Defense against Intracellular Pathogens
Like PMNs and NK cells (in ADCC), cytotoxic T cells, also called CD8+ T cells or cytotoxic T lymphocytes (CTLs), kill infected host cells, but through a different mechanism. By entering cells, intracellular pathogens are protected from antibodies and complement. As such, killing infected host cells is often the only way to attack these invaders. CTLs and NK cells (in ADCC) are important parts of this defense response. The difference between CTLs and NK cells is that CTLs have T cell receptors (TCRs) that are specific to a particular epitope from a microbial antigen. Thus, while NK cells kill host cells infected with a variety of intracellular pathogens (see previous sections and chapter 3), CTLs kill only cells infected with a specific intracellular pathogen (see Figure 3-3).
CTLs have two mechanisms for killing infected cells. In the first, the CTL binds to a microbial antigen displayed on the surface of an infected cell using a TCR on its surface. (How the microbial antigen comes to be displayed on the cell surface will be described later.) This interaction then triggers the CTL to release granules containing two proteins, perforin (a pore-forming cytolysin that pokes holes in the host cell membrane) and proteolytic enzymes (granzymes) that enter the cell through the pore and trigger programmed cell death (apoptosis) in the infected cell ( Figure 4-5). This type of attack kills the infected host cell but not the microbes. Instead, the released microbes are taken up by nearby activated macrophages that are better able to kill them. In the second mechanism, the CTL also releases a second pore-forming cytolysin from the granules, called granulysin. Granulysin is somewhat ineffective in lysing host cells, but it is very effective at killing bacteria. Presumably, granulysin kills bacteria the way perforin kills eukaryotic cells: by creating holes in the bacterial membranes and collapsing the proton motive force that bacteria use to gain energy. Additionally, perforin may also help deliver other yet-to-be-discovered antibacterial lysins or toxins into intracellular compartments of host cells.
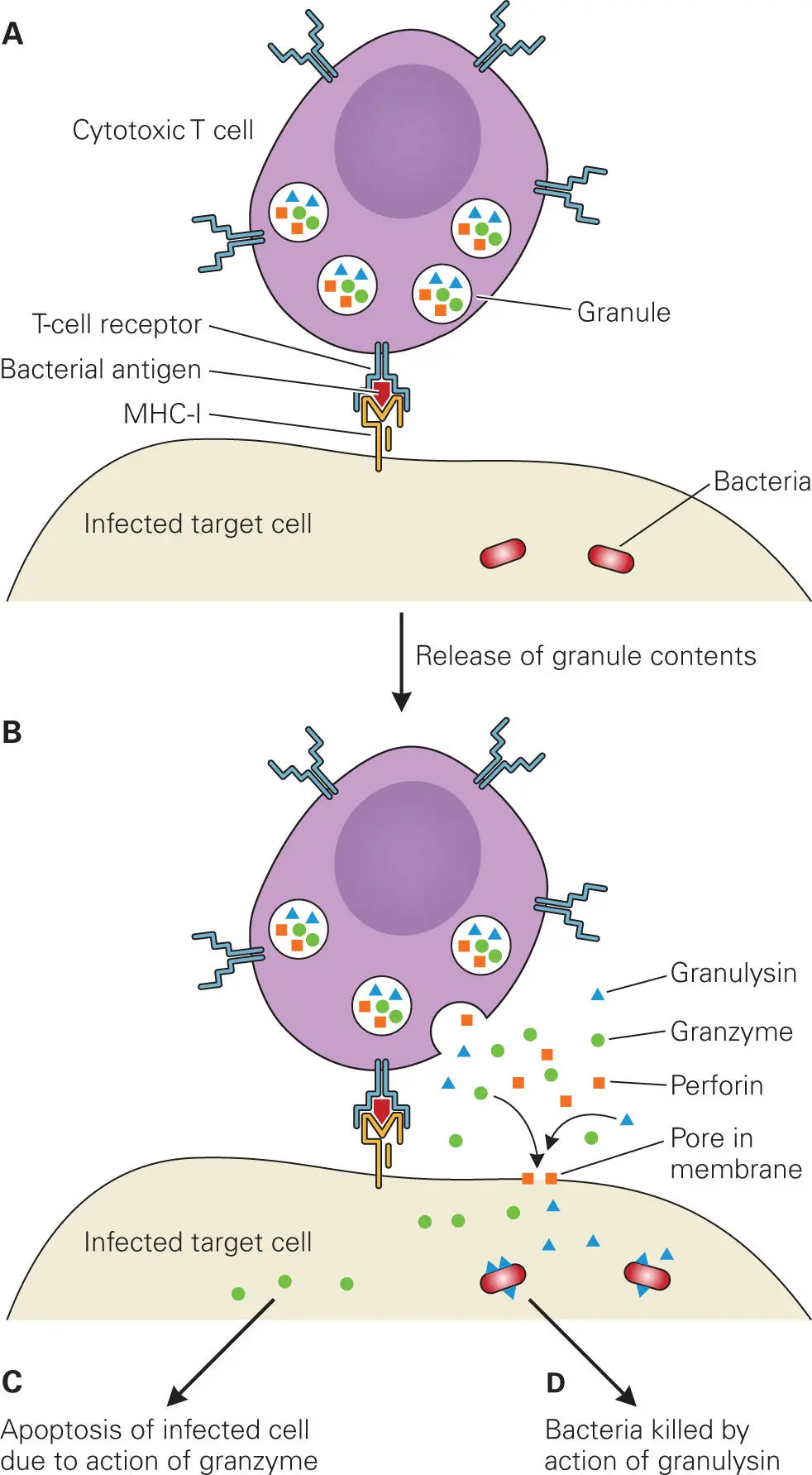
Figure 4-5. Cytotoxic T cell (CTL) action on infected host cells. Cytotoxic T cells (CTLs) mediate their action on infected cells through the release of perforin, granzyme, and granulysin from granules. (A) The cytotoxic T cell recognizes an infected target cell (e.g., macrophage) containing bacteria through T-cell receptor binding to a bacterial antigen displayed via MHC I. (B) The cytotoxic T cell releases granules containing perforin, granzymes, and granulysin. Perforin creates pores in the infected target cell membrane. Granzyme and granulysin enter the target cell through the pores created by perforin. (C) Granzyme triggers signaling pathways that lead to apoptosis of the infected cell. (D) Granulysin binds to and kills intracellular bacteria by creating pores in the bacterial membranes. The infected host cell then processes the degraded bacteria into antigens.
Antigen Presentation to the Immune System
Processing of Protein Antigens by Dendritic Cells
When microbes or their products first enter the body, professional phagocytes called antigen-presenting cells (APCs) engulf, process, and present antigens on their surfaces, which then direct other cells in the adaptive immune system to develop into cells with specific antibacterial activity. There are three types of APCs: dendritic cells (DCs), macrophages, and B cells. As described in chapter 3, macrophages and DCs are important players in the innate immune system, but they also serve as links to the adaptive immune system. Macrophages, as part of the innate immune system, are produced in an immature form (monocytes) and migrate through the bloodstream before moving into tissue where an infection is taking place. They help clear debris from dead human cells that may be circulating in blood or deposited in tissue. Macrophages also play a critical role in initiating and organizing the adaptive immune response.
DCs, like macrophages, initiate and organize the adaptive immune response, but because they are found mainly in localized areas of the dermis, the mucosal lining of the intestinal tract, and lymphoid tissue, they are the first APCs on the scene to process microbial antigens and stimulate the adaptive immune response. B cells can also function as APCs, albeit not as efficiently as macrophages or DCs. In addition to producing and secreting soluble antibodies, B cells also produce membrane immunoglobulins (mIg) that they display on their surfaces. When the mIg captures an antigen, the mIg-antigen complex is internalized and the antigen is processed and presented to the T helper cells.
Peptide Antigens. Figure 4-6shows an overview of how APCs process protein antigens and display the resulting peptide epitopes on their surfaces bound to a protein complex called the major histocompatibility complex (MHC). Two main classes of MHC molecules, MHC I and MHC II, form complexes with peptide epitopes ( Figure 4-7). MHC I molecules are produced by all nucleated cells in the body, while only professional phagocytic APCs, such as DCs, macrophages, B cells, and certain activated epithelial cells, produce MHC II molecules in addition to MHC I molecules. In the case of peptide antigens, the type of MHC used to display the epitope determines the type of immune response that will be elicited (more on this later).
Читать дальше













