Lynne Shore Garcia - Diagnostic Medical Parasitology
Здесь есть возможность читать онлайн «Lynne Shore Garcia - Diagnostic Medical Parasitology» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Diagnostic Medical Parasitology
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:3 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 60
- 1
- 2
- 3
- 4
- 5
Diagnostic Medical Parasitology: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Diagnostic Medical Parasitology»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
Diagnostic Medical Parasitology — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Diagnostic Medical Parasitology», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
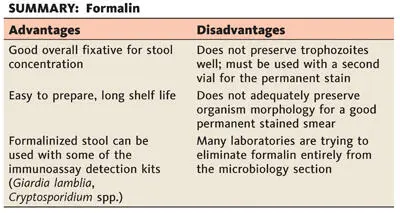
Protozoan cysts (not trophozoites), coccidian oocysts, microsporidian spores, helminth eggs, and larvae are well preserved for long periods in 10% aqueous formalin. Hot (60°C) formalin can be used for specimens containing helminth eggs, since in room-temperature formalin, some thick-shelled eggs (e.g., Ascaris lumbricoides) continue to develop, become infective, and remain viable for long periods. Several grams of fecal material should be thoroughly mixed in 5 or 10% formalin.
To collect large numbers of cysts, eggs, or larvae relatively free from other debris, the whole stool specimen is mixed in water and then strained through several layers of gauze. The suspension is allowed to sediment in a cone-shaped glass or flask for 1 h or more, and the supernatant fluid is discarded. The specimen may be washed several times in this manner before the sediment is finally fixed in hot 10% formalin, as mentioned above. When working with watery diarrhea specimens from patients with suspected cases of coccidiosis or microsporidiosis, the specimen should not be strained through gauze (oocysts and small bits of mucus may cling to the gauze); centrifugation (500 × g for 10 min) is necessary to sediment the oocysts and/or spores.
MIF
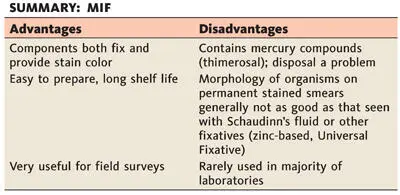
MIF ( 5) is a good stain preservative for most kinds and stages of parasites found in feces; it is especially useful for field surveys. It is used with all common types of stools and aspirates; protozoa, eggs, and larvae can be diagnosed without further staining in temporary wet mounts, either made immediately after fixation or prepared several weeks later. Although some laboratories maintain that a permanent stained smear can be prepared from specimens preserved in MIF, most laboratories using such a fixative examine the material only as a wet preparation (direct smear and/or concentration sediment). For a good discussion of this technique, see reference 27.
The MIF preservative is prepared in two stock solutions, stored separately, and mixed immediately before use.
Solution I (stored in a brown bottle)

Solution II (Lugol’s Solution) (good for several weeks in a tightly stoppered brown bottle)

Combine 9.4 ml of solution I with 0.6 ml of solution II just before use.
1. Add about one-quarter teaspoon (1 g) of fresh feces to the solution, and mix with an applicator stick. Fecal material should be formed or soft if egg counts are to be made later; liquid stool does not work very well for worm burden estimates.
2. Within 24 h, if undisturbed, the specimen forms three well-defined layers. The top layer, a clear orange fluid, consists mainly of formalin, Merthiolate, and water; it does not trap eggs or protozoa. The interface is a thick, pale orange or creamy yellow layer, usually 1 to 2 mm thick; this layer may trap some protozoa and helminth eggs. The bottom layer consists of deeper-staining particulate matter; eggs and protozoa are found throughout this layer.
3. With a glass pipette, MIF direct smears can be made from both the interface and bottom layers. Best results are obtained by making smears from both layers.
4. It has been suggested by some workers that a concentration technique applied to the MIF method (referred to as the MIFC or TFC method) gives satisfactory results. This contention is debatable, and Dunn ( 27) suggests that it is not nearly as reliable as the MIF direct smear method.
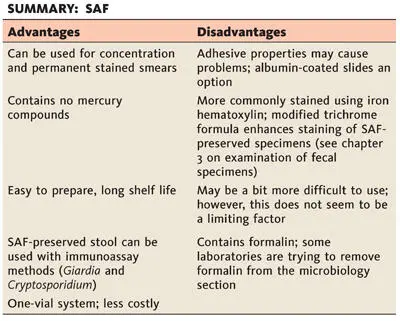
SAF
SAF lends itself to the concentration technique, the permanent stained smear, and fecal immunoassays for Giardia and Cryptosporidium and has the advantage of not containing mercuric chloride, as is found in Schaudinn’s fluid and some of the other fixatives containing PVA ( 20, 21). It is a liquid fixative, much like the 10% formalin described above ( Fig. 2.5). The sediment is used to prepare the permanent smear, and it is frequently recommended that the stool material be placed on an albumin-coated slide to improve adherence to the glass ( Fig. 2.6).
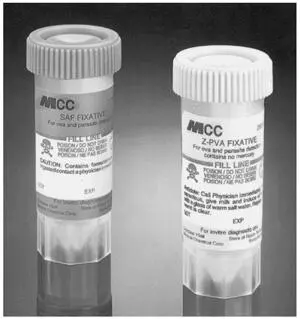
Figure 2.5 Stool collection vials, one containing SAF fixative and the other containing Z-PVA, one of the non-mercury-based fixatives. This combination of collection vials is an excellent option; concentrations and fecal immunoassays can be performed from the SAF vial, while the permanent stained smear can be performed from the Z-PVA vial. doi:10.1128/9781555819002.ch2.f2.5
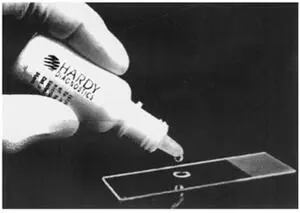
Figure 2.6 Albumin used to precoat the slide prior to the application of SAF-fixed stool concentration sediment. Once the smear is dry, it is ready for permanent staining. doi:10.1128/9781555819002.ch2.f2.6
SAF is considered to be a “softer” fixative than mercuric chloride. The organism morphology is not quite as sharp after permanent staining as that of organisms originally fixed in solutions containing mercuric chloride. The pairing of SAF-fixed material with iron hematoxylin staining provides better organism morphology than does staining SAF-fixed material with trichrome (personal observation). However, a change in the trichrome formulation tends to improve the trichrome staining using SAF (see chapter 3on examination of fecal specimens). Although SAF has a long shelf life and is easy to prepare, the smear preparation technique may be a bit more difficult for less experienced laboratory personnel who are not familiar with fecal specimen techniques. Laboratories that have considered using only a single preservative have selected this option (concentration, permanent stain, fecal immunoassays for Giardia and Cryptosporidium). Helminth eggs and larvae, protozoan trophozoites and cysts, coccidian oocysts, and microsporidian spores are preserved by this method. After centrifugation, special stains for the coccidia (modified acid-fast stains) and the microsporidia (modified trichrome stains) can be used with the concentrate sediment obtained from SAF-preserved stool material.
SAF fixative is prepared as follows:
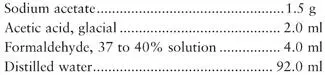
To make Mayer’s albumin, mix equal parts of egg white and glycerin. Place 1 drop on a microscope slide, and add 1 drop of SAF-preserved fecal sediment (from the concentration procedure). After mixing, allow the smear to dry at room temperature for 30 min prior to staining. Mayer’s albumin is also available commercially.
Schaudinn’s Fluid
Schaudinn’s fluid is designed to be used with fresh stool specimens or samples from the intestinal mucosal surface. Many laboratories that receive specimens from in-house patients (no problem with delivery times) often select this approach. Permanent stained smears are then prepared from fixed material. A concentration technique for Schaudinn’s fluid-preserved material is also available but is not widely used.
Читать дальшеИнтервал:
Закладка:
Похожие книги на «Diagnostic Medical Parasitology»
Представляем Вашему вниманию похожие книги на «Diagnostic Medical Parasitology» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Diagnostic Medical Parasitology» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.












