Lynne Shore Garcia - Diagnostic Medical Parasitology
Здесь есть возможность читать онлайн «Lynne Shore Garcia - Diagnostic Medical Parasitology» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Diagnostic Medical Parasitology
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:3 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 60
- 1
- 2
- 3
- 4
- 5
Diagnostic Medical Parasitology: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Diagnostic Medical Parasitology»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
Diagnostic Medical Parasitology — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Diagnostic Medical Parasitology», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
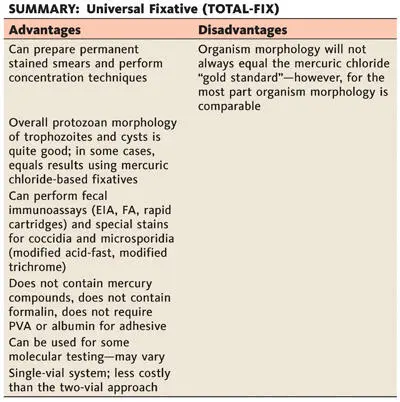

Figure 2.10 TOTAL-FIX is a single-vial collection system; the fixative contains no mercury, no formalin, and no PVA. It is considered a Universal Fixative and can be used for concentrations, permanent stained smears, fecal immunoassays, special stains, and some molecular methods. doi:10.1128/9781555819002.ch2.f2.10
Use of Fixatives
Quality Control for Stool Fixatives
Fixatives for fecal specimens are checked for quality control by the manufacturer before sale, generally with the use of living protozoa. If you prepare your own fixatives, the following approach can be used for quality control. The specimen used for quality control presented below is designed to be used with fixatives from which permanent stained smears will be prepared (Schaudinn’s fluid, Schaudinn’s fluid containing PVA, copper- or zinc-based fixative containing PVA, SAF, MIF, and Universal Fixatives). However, the same quality control specimen can also be used in a concentration; the white blood cells (WBCs) can be seen in the concentrate sediment (sedimentation concentration) or in the surface film (flotation concentration).
1. Obtain a fresh, anticoagulated blood specimen, centrifuge, and obtain a buffy coat sample (try and find a specimen with a high WBC count).
2. Mix approximately 2 g of soft, fresh fecal specimen (normal stool, containing no parasites) with several drops of the buffy coat cells.
3. Prepare several fecal smears, and fix immediately in Schaudinn’s fluid to be quality controlled.
4. Mix the remaining feces-buffy coat mixture in 10 ml of fixatives with or without PVA, SAF, MIF, or Universal Fixative preservative to be quality controlled.
5. Allow 30 min for fixation, and then prepare several fecal smears. Allow to dry thoroughly (60 min at room temperature or 30 to 60 min in an incubator [approximately 35°C]). Do not use a heat block.
6. Stain the slides by the normal staining procedure (trichrome, iron hematoxylin).
7. After staining, if the WBCs appear well fixed and display typical morphology and color, one can assume that any intestinal protozoa placed in the same lot number of preservative would also be well fixed, provided that the fecal sample was fresh and fixed within the recommended time limits.
8. The bulk quality control specimen can be concentrated as for a normal patient specimen. If the fixative is performing correctly, the WBCs will be visible in the concentration sediment or surface film (depending on the method used).
9. Record all quality control results. If the WBC morphology does not confirm good fixation, describe the results and indicate what corrective actions were used (repeated the test, prepared new fixative).
Procedure Notes for Use of Preservatives
1. Most of the commercially available kits have a “fill to” line on the vial label to indicate how much fecal material should be added to ensure adequate preservation of the fecal material (ratio of 1 part stool to 3 parts fixative). However, patients often overfill the vials; remember to open the vials with the vials turned away from your face. There may be excess gas in the vials that may create aerosols once the vial lids are opened.
2. Although the two-vial system (one vial of 5 or 10% buffered formalin [concentration] and one vial of PVA fixative [permanent stained smear]) has always been the “gold standard,” many laboratories are now using other options, including the single-vial collection systems and the Universal Fixative. Changes in the selection of fixatives are based on the following considerations:
A. Problems with disposal of mercury-based fixatives (availability of high-temperature incineration facilities and cost) and lack of multilaboratory contracts for disposal of such products
B. The cost of a two-vial system compared with the cost of a single collection vial
C. Selection of specific stains (trichrome, iron hematoxylin) to use with specific fixatives
D. Whether the newer fecal immunoassay kits can be used with stool specimens preserved in that particular fixative
Procedure Limitations for Use of Preservatives
1. Adequate fixation still depends on the following parameters:
A. Meeting recommended time limits for lag time between passage of the specimen and fixation
B. Use of the correct ratio of specimen to fixative (1:3)
C. Thorough mixing of the fixative and specimen (once the specimen is received in the laboratory, any additional mixing at that time will not counteract the earlier lack of fixative-specimen mixing and contact)
2. Unless the appropriate stain is used with each fixative, the final permanent stained smear may be difficult to examine (organisms hard to see and/or identify). Examples of appropriate combinations are as follows:
A. Schaudinn’s or PVA fixative with trichrome or iron hematoxylin stain
B. SAF fixative with iron hematoxylin stain or the modified trichrome formulation
Shipment of Diagnostic Specimens, Biological Products, Etiologic Agents, or Infectious Substances
Biological materials shipped domestically or internationally must be packaged in compliance with hazardous-materials transport regulations ( 33, 34). The United States has incorporated the United Nations Recommendations on the Transport of Dangerous Goods into law, making the U.S. regulations consistent with foreign shipment regulations. The U.S. regulations for packing diagnostic specimens and infectious agents for shipment were modified in 2006. The Public Health Service, Department of Transportation, and Postal Service specify requirements for packaging and shipping of biological materials. These regulations, plus those for packaging and shipping of materials via air, are similar; most carriers elect to follow the international shipping regulations within the International Air Transport Association (IATA) Dangerous Goods Regulations ( Table 2.5).
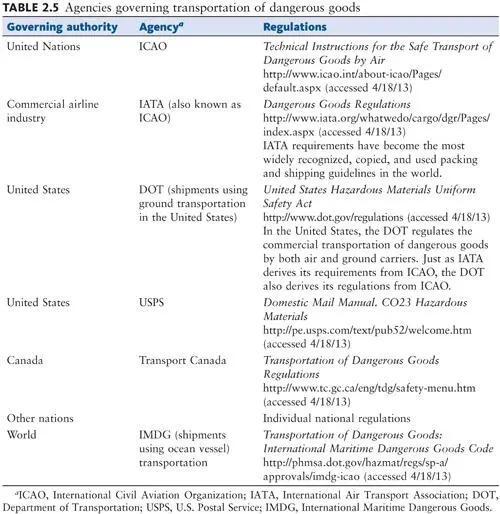
Anyone packaging diagnostic specimens or infectious agents for shipment must receive training and be tested every 2 years. Those who complete the training and test will receive a certificate. The regulations are designed to protect all personnel who may come in contact with the package; compliance with these rules is the responsibility of the sender. Definitions of relevant terms can be found in Tables 2.5and 2.6.
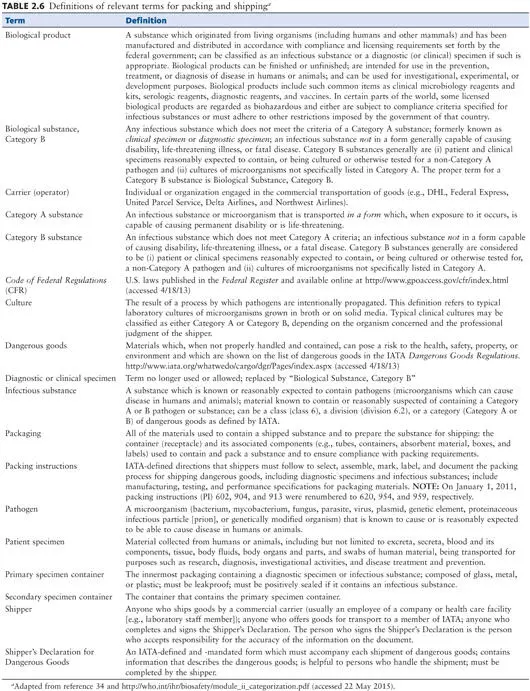
Double mailing containers should be used in shipping any parasitologic specimens other than microscope slides. The inner container is an aluminum screw-cap mailing tube that fits into an outer cardboard screw-cap mailing container. The specimen vials or tubes in the inner aluminum cylinder should be packed in cotton to absorb any moisture or material that might result from leakage or breakage. Instruction sheets, patient information sheets, etc., may be wrapped around the metal cylinder before it is placed in the outer cardboard mailer. For all packages containing infectious substances, an itemized list of contents must be enclosed between the secondary packaging and the outer packaging ( 33, 34). Training and training material for the transportation of dangerous goods and infectious substances are available at the American Society for Microbiology and IATA (training manuals).
Читать дальшеИнтервал:
Закладка:
Похожие книги на «Diagnostic Medical Parasitology»
Представляем Вашему вниманию похожие книги на «Diagnostic Medical Parasitology» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Diagnostic Medical Parasitology» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.












