Lynne Shore Garcia - Diagnostic Medical Parasitology
Здесь есть возможность читать онлайн «Lynne Shore Garcia - Diagnostic Medical Parasitology» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Diagnostic Medical Parasitology
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:3 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 60
- 1
- 2
- 3
- 4
- 5
Diagnostic Medical Parasitology: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Diagnostic Medical Parasitology»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
Diagnostic Medical Parasitology — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Diagnostic Medical Parasitology», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
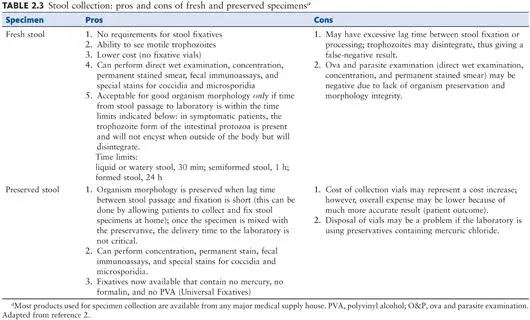
In review, remember that trophozoites only are usually found in liquid specimens, both protozoan trophozoites and cysts can be recovered in soft specimens, and generally cysts only are recovered in formed specimens. The time limits mentioned above are merely guidelines; however, if fresh specimens remain unpreserved for longer times before examination, many if not all organisms may disintegrate or become distorted. Fecal specimens should never be incubated or frozen prior to examination using routine microscopy. When the acceptance criteria for specimen collection are not met, the laboratory should reject the specimen and request additional specimens.
Because there is often a time lag from the time of specimen passage until receipt in the laboratory, many clinicians, clinics, and inpatient wards use a specimen collection system that includes stool preservatives ( Table 2.3). A number of commercial systems are available with many preservative choices; the use of such systems has become routine for many institutions, and some request a custom collection kit that may contain several types of preservatives for stool specimens, depending on the tests normally ordered by the clinicians that they service. Specific information concerning collection kit components is provided in Appendix 1.
KEY POINTS—STOOL SPECIMEN COLLECTION
1. Occupational Safety and Health Act regulations (including standard precautions) should be used for handling all specimens.
2. Interfering substances (oil-based laxatives, barium, antibiotics) should be avoided when stool specimens are collected.
3. Contamination with urine or water should be avoided.
4. Recommendation for collection: three specimens collected, one every other day or within a 10-day time frame.
Note: There are some exceptions; see Table 2.1.
5. Liquid stool: examine or preserve within 30 min of passage (trophozoites). Soft stool: examine or preserve within 1 h of passage (trophozoites and cysts). Formed stool: examine or preserve within 24 h of passage. Note that Dientamoeba fragilis trophozoites can be found in formed stool specimens as well as liquid specimens.
Preservation of Specimens
Preservatives
There are a number of reasons why a lag time may occur from the time of specimen passage until examination in the laboratory (e.g., the workload in the laboratory or the transit distance or time for the specimen to reach the facility). To preserve protozoan morphology and to prevent the continued development of some helminth eggs and larvae, the stool specimens can be placed in preservative immediately after passage (by the patient using a collection kit) or once the specimen is received by the laboratory. There are several fixatives available; the more common ones, including formalin, Merthiolate (thimerosal)-iodine-formalin (MIF), sodium acetate-acetic acid-formalin (SAF), Schaudinn’s fluid (with or without polyvinyl alcohol [PVA]), the single-vial systems, and the Universal Fixative, are discussed in detail ( Table 2.4). Regardless of the fixative selected, adequate mixing of the specimen and preservative is mandatory. A flow diagram for preservation and processing is shown in Fig. 2.3.
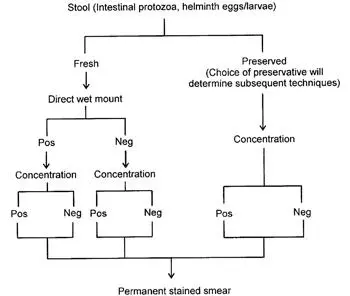
Figure 2.3 Flow diagram for preservation and processing of stool specimens. As mentioned in the text, the examination of fecal specimens using the ova and parasite examination is not considered complete unless a concentration and a permanent stained smear are examined for every specimen submitted to the laboratory. For a fresh specimen, a direct wet mount should be performed if the specimen is very soft to liquid; the complete ova and parasite examination would include the direct wet mount, the concentration, and the permanent stained smear. If the specimen is submitted in preservative, the direct wet mount should be eliminated (no motility is possible); the complete ova and parasite examination would include the concentration and the permanent stained smear ( 1, 2). doi:10.1128/9781555819002.ch2.f2.3
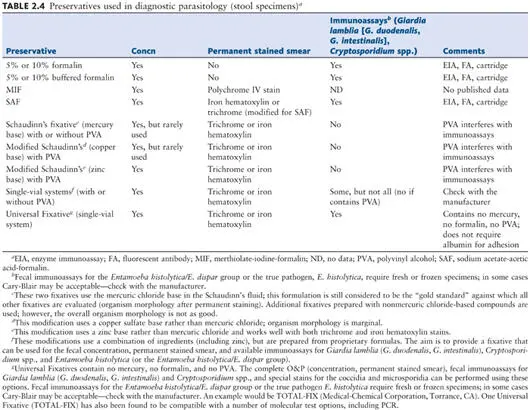
Note When selecting an appropriate fixative, keep in mind that a permanent stained smear is mandatory for a complete examination for parasites ( 1, 2, 26). It is also important to remember that disposal regulations for compounds containing mercury are becoming more restrictive; each laboratory will have to check applicable state and federal regulations to help determine fixative options.
Note It is important to remember that when using fecal immunoassays for the Entamoeba histolytica/E. dispar group or Entamoeba histolytica, fresh or frozen stool is required (in some cases Cary-Blair may be acceptable, but check the package insert prior to use). To date, stool submitted in routine fecal fixatives is not acceptable for these particular fecal immunoassays.
Formalin
Formalin has been used for many years as an all-purpose fixative that is appropriate for helminth eggs and larvae and for protozoan cysts, oocysts, and spores. Two concentrations are commonly used: 5%, which is recommended for preservation of protozoan cysts, and 10%, which is recommended for helminth eggs and larvae. Although 5% is often recommended for all-purpose use, most commercial manufacturers provide 10%, which is more likely to kill all helminth eggs ( Fig. 2.4). To help maintain organism morphology, the formalin can be buffered with sodium phosphate buffers, i.e., neutral formalin. Selection of specific formalin formulations is at the user’s discretion. Aqueous formalin will permit the examination of the specimen as a wet mount only, a technique much less accurate than a permanent stained smear for the identification of intestinal protozoa. However, the fecal immunoassays for Giardia lamblia (G. duodenalis, G. intestinalis) and Cryptosporidium spp. can be performed from the aqueous formalin vial. Fecal mmunoassays for the Entamoeba histolytica/E. dispar group and Entamoeba histolytica are limited to fresh or frozen fecal specimens (some kits may work with stool submitted in Cary-Blair; check the package insert). After centrifugation, special stains for the coccidia (modified acid-fast stains) and the microsporidia (modified trichrome stains) can be performed from the concentrate sediment obtained from formalin-preserved stool material. The most common formalin preparation is 10% formalin, prepared as follows:
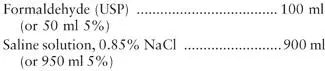
Figure 2.4 When the stool specimen is added to the vial, the final ratio of stool to preservative is about 1:3. doi:10.1128/9781555819002.ch2.f2.4
Dilute 100 ml of formaldehyde with 900 ml of 0.85% NaCl solution. (Distilled water may be used instead of saline solution.)
Note Formaldehyde is normally purchased as a 37 to 40% HCHO solution; however, for dilution, it should be considered to be 100%.
If you want to use buffered formalin, the recommended approach ( 1, 3) is to mix thoroughly 6.10 g of Na 2HPO 4and 0.15 g of NaH 2PO 4and store the dry mixture in a tightly closed bottle. Prepare 1 liter of either 10 or 5% formalin, and add 0.8 g of the buffer salt mixture.
Читать дальшеИнтервал:
Закладка:
Похожие книги на «Diagnostic Medical Parasitology»
Представляем Вашему вниманию похожие книги на «Diagnostic Medical Parasitology» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Diagnostic Medical Parasitology» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.












