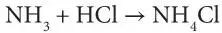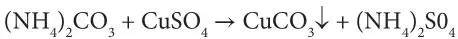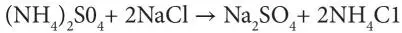In the 1830′s, dental restorative material, called ‘amalgam’ was introduced to the United States. This amalgam was developed in England and France and contained silver, tin, copper, zinc and mercury. The amalgam fillings were not openly embraced by organized dentistry in America, and in 1840, members of the American Society of Dental Surgeons were required to sign pledges not to use mercury fillings. By this time, the current methods of refining metals (including mercury) have been in place. Mercury and its compounds used in dental practice may be responsible for release of mercury into the oral cavity. Compounds of mercury tend to be much more toxic than the element itself, and organic compounds of mercury (e.g., dimethyl-mercury) are often extremely toxic and may be responsible in causing brain and liver damage.
Recently, Wang et al . (2013) conducted an interesting study. They orally administered various doses of cinnabar for 10 consecutive days, then studied the mercury levels. They discovered that the mercury level in serum and tissues are significantly higher than that of vehicle control (Table 2.2). The serum mercury levels in the cinnabar groups were increased in a dose-dependent manner. However, the serum mercury content for the cinnabar group was only about 1/100 of that of the HgCl 2group at the same dose. The mercury levels in the brain tissue of the cinnabar group were raised slowly with the increasing dose and were about 1/19 of HgCl 2group at the same dose. Similar to the pattern of the HgCl 2group, mercury accrued more in kidney than in liver. However, in the HgCl 2group, mercury accumulation was about 330 times higher than that of the cinnabar group.
Table 2.2Mercury contents after cinnabar and HgCl 2administration for 10 days (From Wang et al ., 2013).
| Group |
Serum (ng/ml) |
Brain (ng/g) |
Liver (ng/g) |
Kidney (ng/g) |
| Vehicle |
1.39 ± 0.05 |
2.96 ± 1.24 |
11.19 ± 4.31 |
14.24 ± 2.97 |
| HgCl 20.01 g/kg |
401.94 ± 30.3 |
190.25 ± 11.8 |
5571.91 ± 1211 |
23592.40 ± 446 |
| Cinnabar 0.01 g/kg |
4.10 ± 0.47 |
10.63 ± 2.53* |
25.58 ± 5.97 |
70.00 ± 18.02 |
| Cinnabar 0.05 g/kg |
14.63 ± 0.59 |
11.07 ± 2.10 |
32.73 ± 6.96 |
82.69 ± 20.02 |
| Cinnabar 0.1 g/kg |
26.75 ± 6.98 |
12.20 ± 1.44 |
84.75 ± 9.47 |
271.10 ± 49.25 |
| Cinnabar 1 g/kg |
75.30 ± 9.24 |
13.27 ± 2.22 |
89.47 ± 10.02 |
455.88 ± 76.93 |
Meanwhile, there were no significant differences in the tissue distribution patterns between the cinnabar and pure HgS groups (Table 2.3) except that the pure HgS group accumulated mercury in the kidney ~2 times higher than that of the cinnabar group.
Table 2.3Mercury contents after cinnabar and HgS administration for 10 days (From Wang et al ., 2013).
| Group |
Serum (ng/ml) |
Brain (ng/g) |
Liver (ng/g) |
Kidney (ng/g) |
| Vehicle |
1.52 ± 0.02 |
1.83 ± 0.49 |
5.71 ± 1.69 |
16.29 ± 1.19 |
| Cinnabar 0.1 g/kg |
24.62 ± 1.55 |
12.12 ± 1.19 |
77.57 ± 10.17 |
206.21 ± 33.76 |
| HgS 0.1 g/kg |
27.44 ± 3.29 |
8.03 ± 1.98 |
41.39 ± 9.78 |
454.56 ± 70.68 |
This study indicates that cinnabar is remarkably different from HgCl 2in mercury absorption and tissue distribution. This finding is profound because up until recently synthetic chemicals were considered to be the same as natural chemicals (Khan and Islam, 2016). As will be discussed in latter chapters, this marks a bifurcation point in terms of natural chemicals following a different pathway from artificial chemicals.
Sal ammoniac is a naturally occurring substance, mainly containing NH 4Cl. It is the best known of the ammonium-bearing minerals. It forms in natural fumaroles, where gas vents from underground from volcanic activity. It also forms from the process of the burning of coal in coal deposits. The formation of Sal ammoniac is unique, as it is created from sublimation, meaning it crystallizes directly from gaseous fumes and bypasses a liquid phase. Sal ammoniac is highly soluble in water. It is known for its utility in many applications, ranging from fertilizer, to colouring agent to medical usage.
Sal ammoniac (naturally occurring Ammonium Chloride) has reported to be first found in the wastelands of Central Asia, from which it was used by Muslim Alchemists of the medieval age, primarily for distillation of organic materials (Multhauf, 1965). In later centuries, there is evidence that Sal ammoniac was being produced through solar distillation of camel urine and other organic products, in proportion of five parts urine, one part common salt, and one-half part soot, which was also derived from natural products (Malthauf, 1965). Soot in general is a common source of heavy metals. Whenever soot is formed, they contain heavy metals that act as the nucleation site within the powdery soot. It is also reported that soot collected from the chimney of camel dung furnaces in Egypt contained natural ammonium chloride. This is expected as any herbivorous animal would have natural supply of salt in its diet. The concept of combining ammonia (then known as volatile alkali) and hydrochloric acid (then known as ‘spirit of salt’) wasn’t invented until late 18 thcentury (Malthauf, 1965). The now well-known synthesis process was:
(2.1) 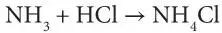
This synthesis process was deemed to be cheaper than the ‘decomposition, which used decomposition of soot (including organic material), sulfuric acid and salt. By the mid-nineteenth century the synthetic process had superseded all others for the manufacture of sal ammoniac, and it was accomplished with utter simplicity through the addition of hydrochloric acid to the “ammonia liquor”, which was a residue from coal distillation.
Later on, another process evolved. It involved double decomposition of ammonium sulfate and sodium chloride:
 (2.2) 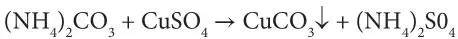
(2.3) 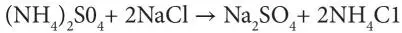
Ammonium carbonate refers to smelling salts, also known as ammonia inhalants, spirit of hartshorn or sal volatile. Today, this chemical is known as baker’s ammonia – the source of gaseous ammonia. It is also the form taken by ammonia when distilled from carbonaceous material without drying. Similarly, copper sulfate can be derived from naturally occurring blue vitriol, other sulfates, such as calcium sulfate (gypsum in its natural state). In Equation 2.2, the requirement is that an insoluble carbonate be formed, permitting the separation of the ammonium sulfate. The separation of the sal ammoniac in the second reaction was accomplished either by its sublimation or by differential crystallization.
Even later, came the double decomposition of ammonium carbonate and magnesium chloride (bittern), following the reaction:
Читать дальше