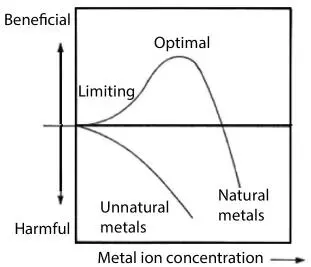
Figure 2.2The usefulness of metal depends on its concentration as well as source
(Figure adapted from Islam et al ., 2016 and Weber and Rutala, 2001).
In ancient times, only the upper graph of Figure 2.2 Existed because all processes were sustainable from both mass and energy perspectives (Khan and Islam, 2016). Today, it has become a common practice to lump chemicals that had been in use since the ancient time with those discovered in later era, for which there is no longer a natural processing technique available. For instance, many clinical studies have lumped cadmium, lead, mercury, thallium, bismuth, arsenic, antimony and tin in the same vein. Yet, cadmium was discovered in 1872 (just before the invention of electricity in 1979), thallium was discovered in 1861 whereas other metals were either used in their natural form (ore) or processed through natural means (e.g. open fire, natural additives). The first metal to be smelted in the ancient Middle East was probably copper (by 5000 BCE), followed by tin, lead, and silver. To achieve the high temperatures required for smelting, furnaces with forced-air draft were developed; for iron, temperatures even higher were required. Smelting thus represented a major technological achievement. Charcoal was the universal fuel until coke was introduced in 18th-century England (Islam et al ., 2010).
Many forms of mercury exist in nature, including elemental mercury, inorganic mercury, and organic mercury (Weber and Rutala, 2001). From ancient times onward, organic mercury 1compounds have been used as antiseptics, antibacterials, fungicides and other disinfectants. These are highly toxic beyond very low concentration. It means their optimal concentration (Figure 2.2) is quite low. Beyond the optimal concentration, organic mercury can produce toxicity through skin absorption, ingestion, or inhalation. They are also associated with gastrointestinal, renal, and neurologic toxicity. Thimerosal (merthiolate; 2(9-ethylmerucrio-thio) benzoic acid sodium salt has been widely used as a bactericide at concentrations of 0.001% to 0.1%. It has also been used as preservatives in pharmaceuticals and cosmetics, including in vaccines, eyedrops, contact lens cleaning and storage solutions, cosmetic creams, toothpaste, mouthwash, etc. (Van’t Veen and Joost, 1994). While this is widely recognized, few are aware of the fact that the chemicals that are used in modern industry are not chemicals that are naturally occurring, they are instead synthetic. Therefore, they are toxic even below the optimal concentration, meaning the lower graph of Figure 2.2 Should be consulted. This same principle also applies to mercury vapour. For instance, today’s mercury vapor lamps use an arc through vaporized mercury in a high-pressure tube to create very waves (of various wavelengths) directly from its own arc. This is different from fluorescent lightbulbs, which use the mercury vapor arc to create a weaker light that mainly creates UV light to excite the phosphors. This usage is just one of many alterations of original usage of mercury.
In Figure 2.3, relative output spectra of low- and medium pressure mercury arc lamps are shown. The ‘medium pressure’ refers to lamps for which the internal pressures are in the range of 2 to 5 bar and that operate at temperatures ranging from 700-900 C. The important aspect of the figure is the fact that UV output is a strong function of internal pressure of the lamp generate characteristic wavelengths of a broad spectrum. With conventional mass and energy balance treatment that disconnects the transition between mass and energy such dependence of relative irradiance on pressure cannot be quantified or predicted qualitatively. However, the technique (using the ‘galaxy’ model) proposed by Islam (2014) and later used by Khan and Islam (2016) makes it possible to account for alteration in the subatomic level to be coupled with tangible expression, such as light intensity. The next feature of this figure is the fact that different irradiance level of UV will kill different types of bacteria. Once again, such antibacterial effects can be described with the galaxy model that allows for different wavelengths to destroy different types of bacteria (based on their characteristic length). 2
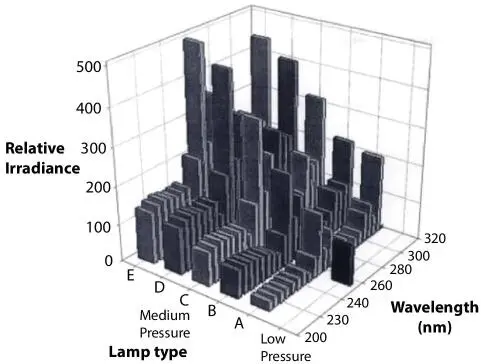
Figure 2.3Relative output spectra of low- and medium pressure mercury arc lamps in the germicidal UV range. A=2 kW, 100 W/in; B=3.5 kW, 150 W/in; C=5 kW, 150 W/in; D= 6.4 kW, 150 W/in; E=7 kW, 200 W/in (from Blachley III and Peel, 2001).
Knowledge of cinnabar (HgS) is traced back to ancient Assyria and Egypt, but also to China (Wang, 2015). It had value for both medicinal and alchemy applications. In traditional Chinese medicine (TCM), cinnabar has been a high value medicinal component. Wang (2015) pointed out Shennong’s Classic of Materia Medica claims that cinnabar can treat practically all ailments involving the five yang organs 3, namely, heart, liver, spleen, lung and kidney. Cinnabar reportedly has calming and revitalizing effects, which help build one’s strength and improve vision, and kill “evil spirits”. The term “evil spirit” has been known to imply inexplicable ailments, including mental illness (Islam et al ., 2017). There have been reports of improvement of lungs and hearts owing to ‘moistening actions’ of cinnabar while consumed orally or even applied externally. Most significantly, cinnabar was known to be a cure of convulsion and epilepsy, as well as fetal toxicity and pox virus. It was also considered to prevent malaria (Wang, 2015).
One such compound is mercury sulphide (cinnabar), which is known in ancient Chinese (called zhūshā, 朱砂), Greek and Arabic (called zinjafar, 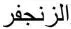 ) culture with universal use in medicine as well as general alchemy. Cinnabar has been used in traditional Chinese medicine as a sedative for more than 2000 years (Huanga et al ., 2007). In addition to being used for insomnia, cinnabar is thought to be effective for cold sores, sore throat, and some skin infections.
) culture with universal use in medicine as well as general alchemy. Cinnabar has been used in traditional Chinese medicine as a sedative for more than 2000 years (Huanga et al ., 2007). In addition to being used for insomnia, cinnabar is thought to be effective for cold sores, sore throat, and some skin infections.
Cinnabar is generally found in a massive, granular or earthy form and is bright scarlet to brick-red in color, has the highest refractive index of any mineral (King, 2002).
It turns out that mercury compounds continued to be used in Europe during 15 ththrough 20 thcentury. In the era before synthetic antibiotics, sexually-transmitted diseases were of great concern. In search for a cure, various forms of mercury were tried. As such, mercury was the remedy of choice for syphilis in Protestant Europe. Paracelsus (1493-1541) formulated mercury as an ointment because he recognised the toxicity and risk of poisoning when administrating mercury as an elixir. Mercury was already being used in Western Europe to treat skin diseases.
The dominating medical use of Hg, (in metallic form and as calomel, Hg 2Cl 2), in Sweden in the second half of the 19th century indicates that some persons were highly exposed to Hg, mainly for treatment of syphilis, and 0.3-1% of the population of 3.5-5 millions were treated for venereal diseases (10,000-50,000 patients).
Sublimate (HgCl 2) is in certain countries still used as an antiseptic for wounds. It was used in large quantities during the World Wars, triggered by the largely increased use of Hg in explosives. Sublimate was also used for preserving wood.
Читать дальше



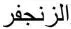 ) culture with universal use in medicine as well as general alchemy. Cinnabar has been used in traditional Chinese medicine as a sedative for more than 2000 years (Huanga et al ., 2007). In addition to being used for insomnia, cinnabar is thought to be effective for cold sores, sore throat, and some skin infections.
) culture with universal use in medicine as well as general alchemy. Cinnabar has been used in traditional Chinese medicine as a sedative for more than 2000 years (Huanga et al ., 2007). In addition to being used for insomnia, cinnabar is thought to be effective for cold sores, sore throat, and some skin infections.










