1 ...6 7 8 10 11 12 ...21 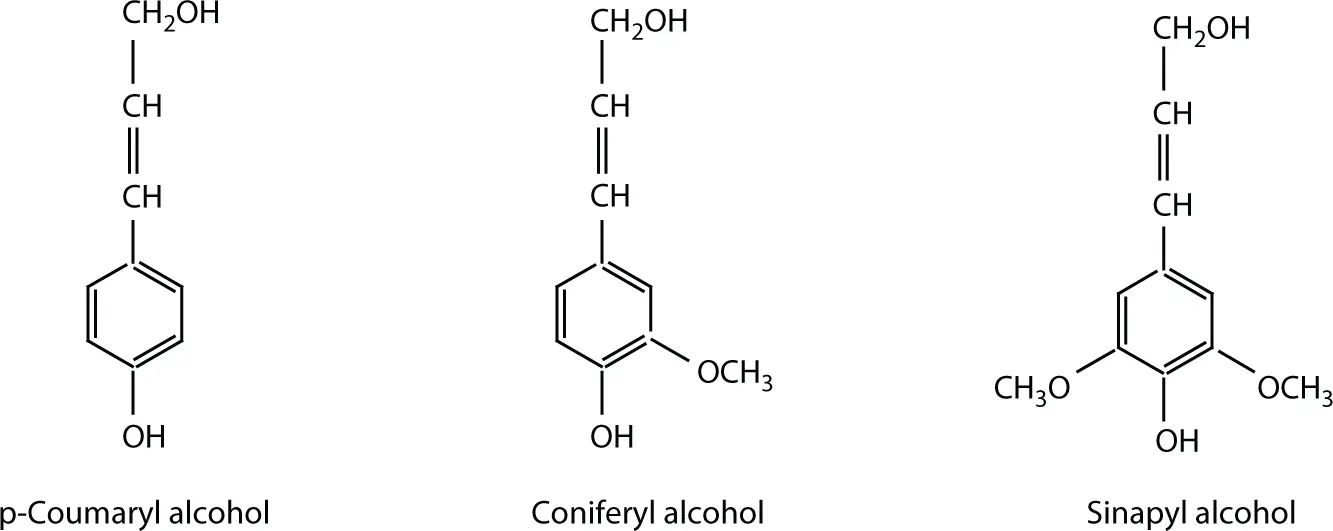
Figure 1.4 The three monolignols.
Thus, the monolignols are the building blocks of the lignin macromolecule. Lignin is therefore defined as an amorphous polyphenolic material arising from an enzyme-mediated dehydrogenative polymerization (DHP) of three phenylpropanoid monomers, coniferyl, synapyl, and p -coumaryl alcohols.
The following nomenclature of radicals ( Figure 1.5) and the building units from which lignin is derived should be kept in mind to understand scientific publications on lignin:
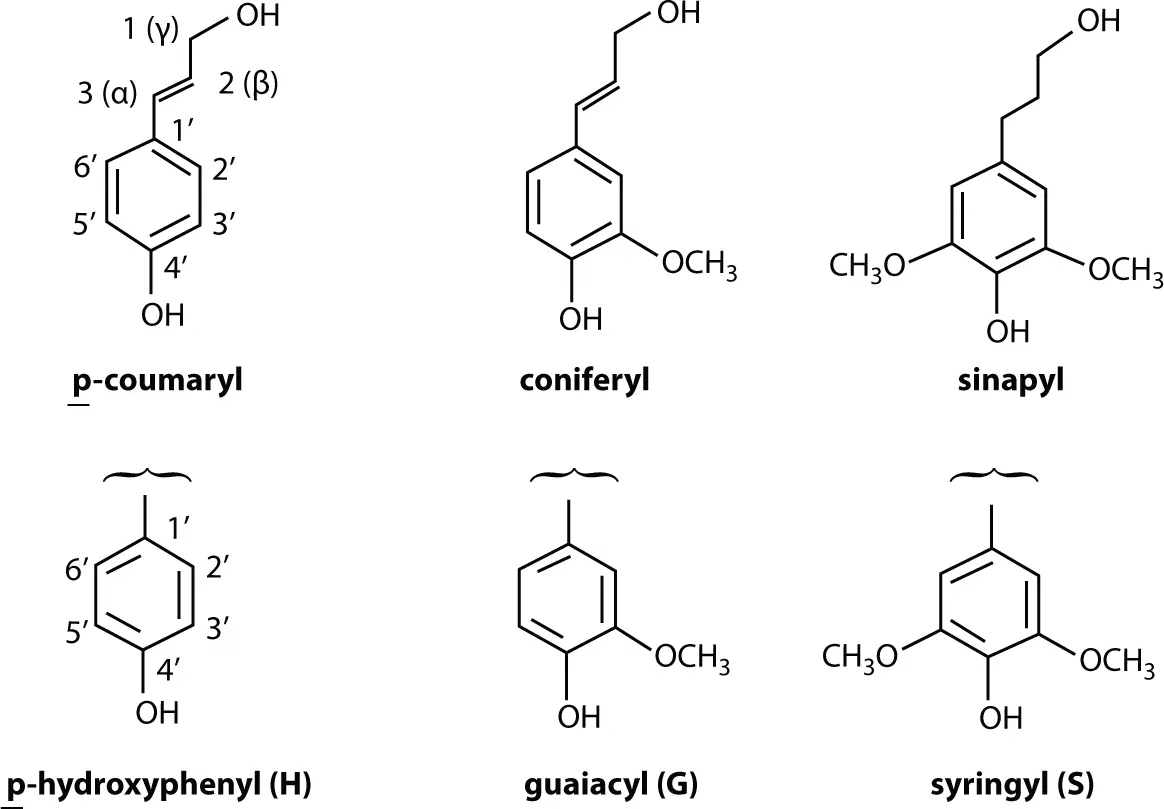
Figure 1.5 Radicals and units—nomenclature.
Lignin’s functions in the tree are as follows (Wang Wood Chemistry Class):
1 Support for mechanical strength
2 Antioxidant for protection
3 Sealant and reinforcing agents, bonding cellulose and hemicellulose together
4 Cross-linker to cross-link he polymeric carbohydrates
The so-called lignin–carbohydrates complex defines the type of covalent bonds existing between lignin and hemicelluloses. These are mainly benzyl ester and benzyl ether linkages between the side chains of xylans and phenyl glycosidic linkages with the main chain of glucomannans [11].
Isolation of lignin in an unchanged form (native lignin) is rendered difficult due to its complex structure and its location within the cell wall. Hence, determination of the exact chemical structure of lignin is therefore difficult [11]. All methods of isolation have the disadvantage of either fundamentally changing the native structure of lignin or releasing only a part of it in a relatively unchanged condition.
There are two methods by which lignin can be isolated from extractive free wood [19]:
1 As an insoluble residue after hydrolytic removal of the polysaccharides.
2 Alternatively, lignin can be hydrolyzed from wood or converted into soluble derivative.
According to method (1) the polysaccharides can be removed in the following procedures:
1 Klason lignin: Klason lignin is obtained after removing the polysaccharides from extractives-free wood by hydrolysis with 72% sulfuric acid.
2 Cellulolytic enzymes may be used to dissolve polysaccharides from finely divided wood meal leaving behind lignin as residue. This lignin is called cellulolytic enzyme lignin (CEL). This method is tedious but the CEL retains its original structure essentially unchanged.
Bjőrkman lignin, also called milled wood lignin (MWL), has been widely used for structural studies. Wood meal is ground in a ball mill either without any solvent or with a solvent such as toluene, which is a non-swelling solvent. The lignin can then be extracted by using a mixture of dioxane, water, and HCl (dioxane lignin). Lower temperature extraction minimizes structural changes of lignin.
A number of researchers have tried the dioxane method in lower temperature (and consequently lower yields) to minimize structural changes in extracted lignins [4].
For the extraction of lignins, a modified dioxane method and ionic liquid and comparative molecular weight (MW) and structural studies by chromatography and 13C NMR spectroscopy techniques were used [20].
In recent years, ionic liquids have been used to dissolve carbohydrates, and lignin residue is hoped to be relatively unchanged [21].
The second method involves the formation of soluble lignin derivatives, namely, lignosulfonate.
1.4.3.2 Functional Groups in Lignin
Lignin contains characteristic methoxyl groups, phenolic hydroxyl groups, and some terminal aldehyde groups in the side chains. Only relatively few of the phenolic hydroxyls are free; most of them are occupied by linkages to neighboring phenylpropane units. The syringyl units in hardwood lignin are extensively etherified. Alcoholic hydroxyl groups and carbonyl groups are introduced into the final lignin polymer during the dehydrogenative polymerization process.
1.4.3.3 Evidences for the Phenylpropane Units as Building Blocks of Lignin
The following methods based on classical organic chemistry, namely, degradation and synthesis, led to the conclusion, already by 1940, that lignin is built up by phenylpropane units [11].
1 Permanganate oxidation (methylated spruce lignin) affords veratric acid (3,4-dimethoxybenzoic acid) and minor amounts of isohemipinic (4,5-dimethoxyisophthalic) acid and dehydrodiveratric acid. The formation of isohemipinic acid supports occurrence of condensed structures (e.g., β-5 or γ-5). See structures 1 to 3 in Figure 1.6.
2 Nitrobenzene oxidation of softwoods in alkali results in the formation of vanillin (4-hydroxy-3-methoxybenzaldehyde). Oxidation of hardwoods and grasses results respectively in syringaldehyde (3,5 dimethoxy-4-hydroxybenzaldehyde) and p-hydroxybenzaldehyde. See structures 4 to 6 in Figure 1.6.
3 Hydrogenolysis yields propylcyclohexane derivatives. See structure 7 in Figure 1.6.
4 Ethanolysis yields so-called Hibbert ketones. See structures 8 to 11 of Figure 1.6.
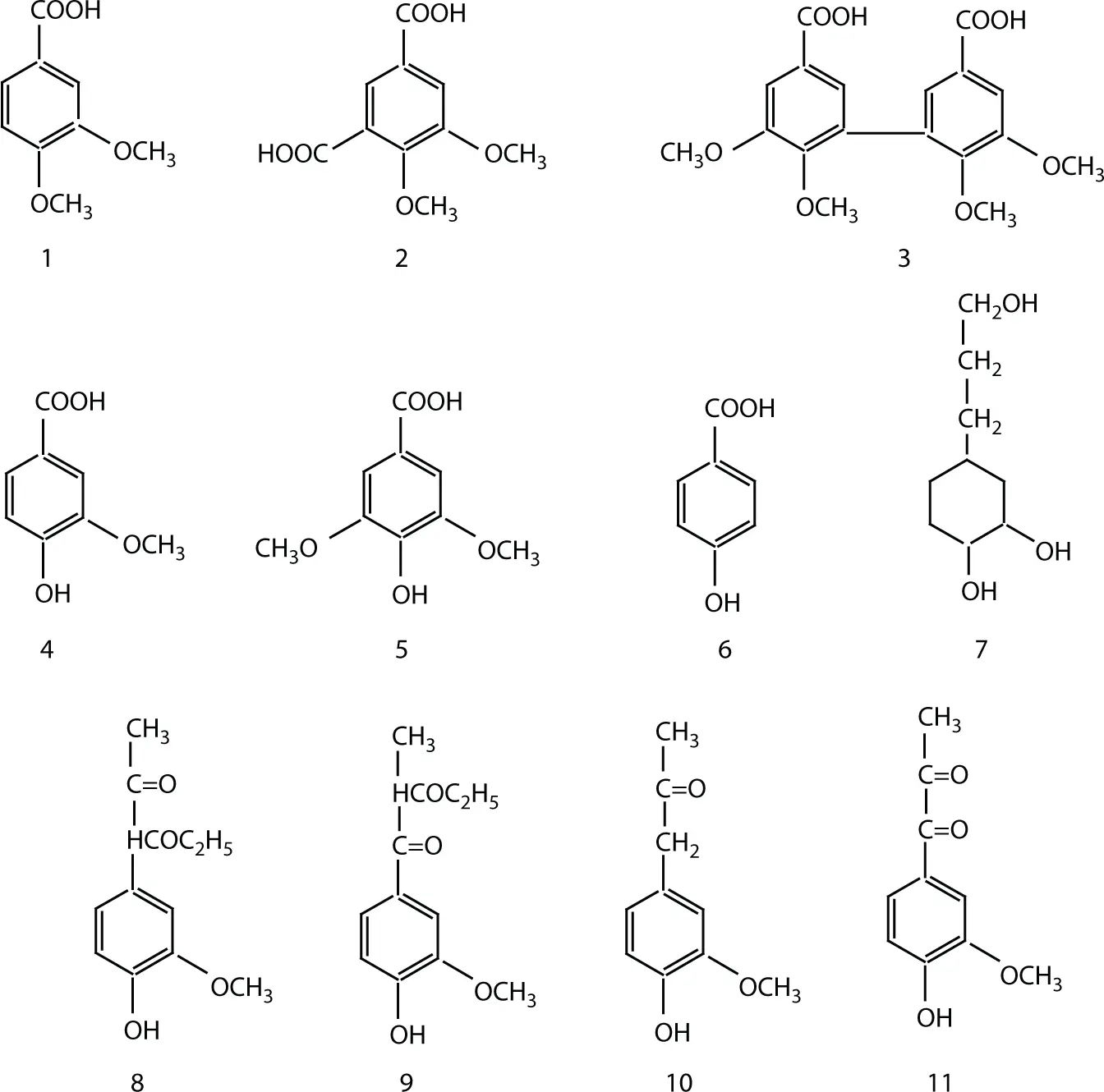
Figure 1.6 Various degradation products of lignin.
1.4.3.4 Dehydrogenation Polymer (DHP)
The biosynthesis of lignin from the monomeric phenylpropane units can be generally described as a dehydrogenative polymerization. The principal ideas about such a pathway were elaborated by Freudenberg and co-workers [11]. They were the first to produce in vitro lignin called dehydrogenation polymer (DHP) by treating coniferyl alcohol with a fungal laccase from the mushroom Psalliota campestris or with a horseradish peroxidase by hydrogen peroxide.
The first step of the biochemical pathway for building up lignin macromolecules is the enzymatic dehydrogenation of p -hydroxycinnamyalcohols, yielding mesomeric ring systems with a loosened proton. Figure 1.7shows the formation of phenoxy radicals from coniferyl alcohol by a one-electron transfer:
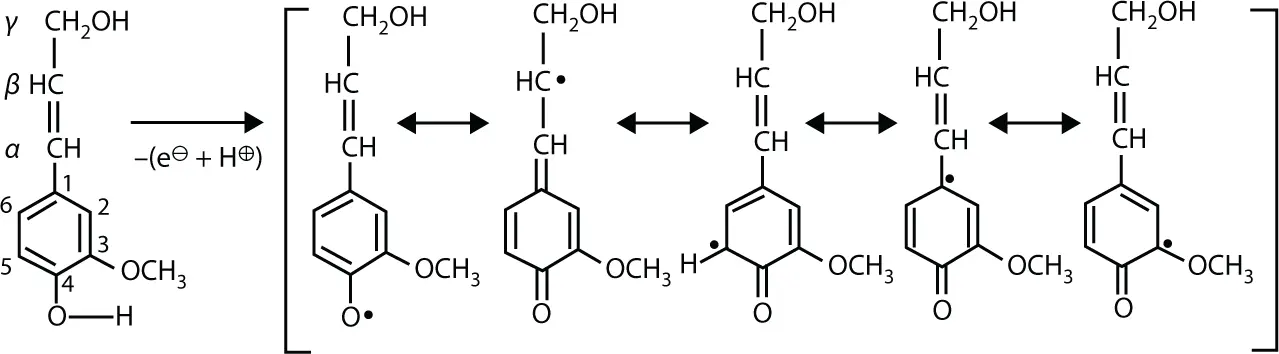
Figure 1.7 Enzymatic dehydrogenation of coniferyl alcohol yielding phenoxy radicals.
The origin of the hydrogen peroxide was cleared up by discovering cell-wall-bound enzyme systems able to deliver H 2O 2[22, 23].
Only 4-phenoxyradical I to IV are actually involved in lignin biosynthesis. Structure V is sterically hindered or thermodynamically not favored [24].
The polymerization of monomeric precursors by random coupling reactions cannot be studied in vivo, but it is known from numerous in vitro experiments to run without enzymatic control as a spontaneous process. The first step in polymerization is the formation of dimeric structures. Some prominent lignol dimers called dilignols are shown in Figure 1.8.
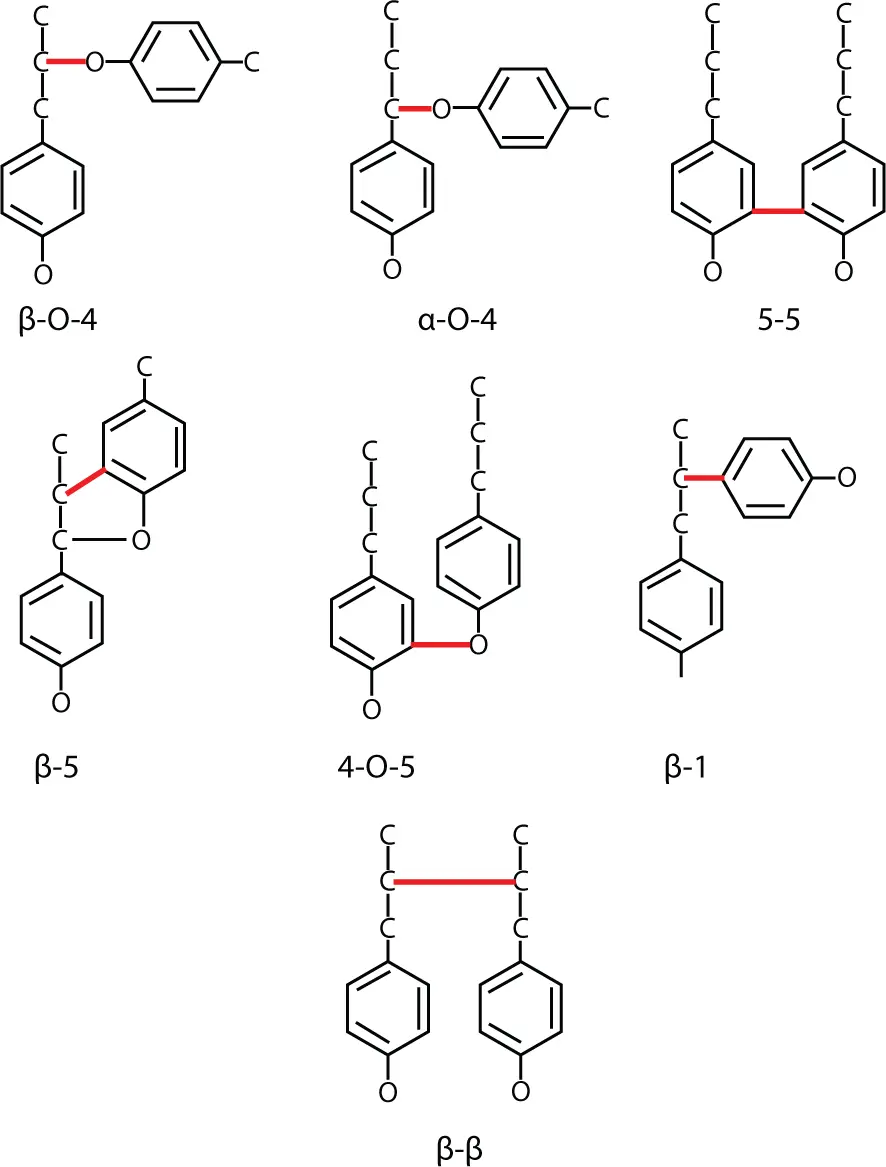
Figure 1.8 Typical dilignol structures [25].
Читать дальше

















