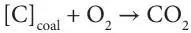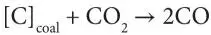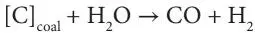The aim of underground (or in situ ) gasification of coal is the conversion into combustible gases by combustion of a coal seam in the presence of air, oxygen, or oxygen and steam. Thus, seams that were considered to be inaccessible, unworkable, or uneconomical to mine could be put to use. In addition, strip mining and the accompanying environmental impacts, the problems of spoil banks, acid mine drainage, and the problems associated with use of high-ash coal are minimized or even eliminated.
The principles of underground gasification are very similar to those involved in the above-ground gasification of coal. The concept involves the drilling and subsequent linking of two boreholes so that gas will pass between the two (King and Magee, 1979). Combustion is then initiated at the bottom of one bore-hole (injection well) and is maintained by the continuous injection of air. In the initial reaction zone (combustion zone), carbon dioxide is generated by the reaction of oxygen (air) with the coal:
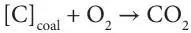
The carbon dioxide reacts with coal (partially devolatilized) further along the seam (reduction zone) to produce carbon monoxide:
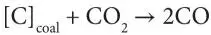
In addition, at the high temperatures that can frequently occur, moisture injected with oxygen or even moisture inherent in the seam may also react with the coal to produce carbon monoxide and hydrogen:
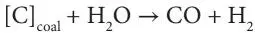
The gas product varies in character and composition but usually falls into the low-heat (low Btu) category ranging from 125 to 175 Btu/ft 3(King and Magee, 1979).
2.5.1 Feedstock Pretreatment
While feedstock pretreatment for introduction into the gasifier is often considered to be a physical process in which the feedstock is prepared for gasifier – typically as pellets or finely ground feedstock – there are chemical aspects that must also be considered. Some feedstocks, especially certain types of coal, display caking, or agglomerating, characteristics when heated (Speight, 2013) and these coal types are usually not amenable to treatment by gasification processes employing fluidized-bed or moving-bed reactors; in fact, caked coal is difficult to handle in fixed-bed reactors. The pretreatment involves a mild oxidation treatment which destroys the caking characteristics of coals and usually consists of low-temperature heating of the coal in the presence of air or oxygen.
While this may seemingly be applicable to coal gasification only, this form of coal pretreatment is particularly important when a non-coal feedstock is co-gasified with coal. Co-gasification of other feedstocks, such as coal and especially biomass, with crude oil coke offers a bridge between the depletion of crude oil stocks when coal is used as well as a supplementary feedstock based on renewable energy sources (biomass). These options can contribute to reduce the crude oil dependency and carbon dioxide emissions since biomass is known to be neutral in terms of carbon dioxide emissions. The high reactivity of biomass and the accompanying high production of volatile products suggest that some synergetic effects might occur in simultaneous thermochemical treatment of petcoke and biomass, depending on the gasification conditions such as: (i) feedstock type and origin, (ii) reactor type, and (iii) process parameters (Penrose et al. , 1999; Gray and Tomlinson, 2000; McLendon et al. , 2004; Lapuerta et al. , 2008; Fermoso et al. , 2009; Shen et al. , 2012; Khosravi and Khadse, 2013; Speight, 2013, 2014a, 2014b; Luque and Speight, 2015).
The first step in the process is feedstock preparation which may include unit operations such as (i) size reduction, (ii) screening, and (iii) slurrying. The design of the feedstock preparation section depends on the properties of the raw material feed and on the requirements of the gasification technology that is selected. For example, preparing any form of crude oil resid, biomass, or waste for gasification is very different from the preparation of bituminous coal for the gasifier, and the type of gasifier suitable for the gasification may also (or more than likely) be feedstock dependent ( Chapter 12).
For example, carbonaceous fuels are gasified in reactors, a variety of gasifiers such as the fixed- or moving-bed, fluidized-bed, entrained-flow, and molten-bath gasifiers have been developed (Shen et al. , 2012; Speight, 2014b). If the flow patterns are considered, the fixed-bed and fluidized-bed gasifiers intrinsically pertain to a countercurrent reactor in that fuels are usually sent into the reactor from the top of the gasifier, whereas the oxidant is blown into the reactor from the bottom. With regard to the entrained-flow reactor, it is necessary to pulverize the feedstock (such as coal and petcoke). On the other hand, when the feedstock is sent into an entrained-flow gasifier, the fuels can be in either form of dry feed or slurry feed. In general, dry-feed gasifiers have the advantage over slurry-feed gasifiers in that the former can be operated with lower oxygen consumption. Moreover, dry-feed gasifiers have an additional degree of freedom that makes it possible to optimize synthesis gas production (Shen et al. , 2012).
2.5.2 Feedstock Devolatilization
The devolatilization (or pyrolysis) process commences at approximately 200 to 300°C (390 to 570°F), depending upon the nature and properties of the feedstock. Volatiles are released and a carbonaceous residue (char) is produced, resulting in up to 70% weight loss for many feedstocks. The process determines the structure and composition of the char, which will then undergo gasification reactions.
In a gasifier, the feedstock particle is exposed to high temperatures generated from the partial oxidation of the carbon. As the particle is heated, any residual moisture (assuming that the feedstock has been pre-fired) is driven off and further heating of the particle begins to drive off the volatile gases. Discharge of the volatile products will generate a wide spectrum of hydrocarbon derivatives ranging from carbon monoxide and methane to long-chain hydrocarbon derivatives comprising tars, creosote, and high-boiling oil. The complexity of the products will also affect the progress and rate of the reaction when each product is produced by a different chemical process at a different rate. At a temperature above 500 oC (930 oF) the conversion of the feedstock to char and ash and char is completed. In most of the early gasification processes, this was the desired byproduct but for gas generation the char provides the necessary energy to effect further heating and – typically, the char is contacted with air or oxygen and steam to generate the product gases. Furthermore, with an increase in heating rate, feedstock particles are heated more rapidly and are burned in a higher temperature region, but the increase in heating rate has almost no substantial effect on the mechanism (Irfan, 2009).
The gasification process occurs as the char reacts with gases such as carbon dioxide and steam to produce carbon monoxide and hydrogen. Also, corrosive ash elements such as chloride and potassium may be refined out by the gasification process, allowing the high temperature combustion of the gas from otherwise problematic feedstocks. Although the initial gasification stage is completed in seconds or even less at elevated temperature, the subsequent gasification of the char produced at the initial gasification stage is much slower, requiring minutes or hours to obtain significant conversion under practical conditions and reactor designs for commercial gasifiers are largely dependent on the reactivity of the char, which in turn depends on the nature of feedstock. The reactivity of char also depends upon parameters of the thermal process required to produce the char from the original feedstock. The rate of gasification of the char decreases as the process temperature increases due to the decrease in active surface area of char. Therefore a change of char preparation temperature may change the chemical nature of char, which in turn may change the gasification. The reactivity of char may be influenced by catalytic effect of mineral matter in the char.
Читать дальше