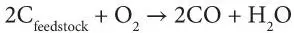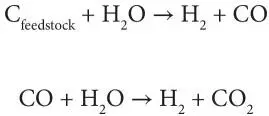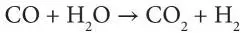Heat and mass transfer processes in fixed- or moving-bed gasifiers are affected by complex solids flow and chemical reactions. Moving-bed gasifiers are countercurrent flow reactors in which the feedstock enters at the top of the reactor and oxygen (air) enters at the bottom of the reactor. Because of the countercurrent flow arrangement of the reactor, the heat of reaction from the gasification reactions serves to pre-heat the coal before it enters the gasification reaction zone. Consequently, the temperature of the synthesis gas exiting the gasifier is significantly lower than the temperature needed for complete conversion of the feedstock. However, coarsely crushed feedstock may settle while undergoing (i) thermal drying, (ii) pyrolysis-devolatilization, (iii) gasification, and (iv) reduction. In addition, the particles change in diameter, shape, and porosity – non-ideal behavior may result from bridges, gas bubbles, channeling, and a variable void fraction may also change heat and mass transfer characteristics.
Gasification involves the thermal decomposition of feedstock and the reaction of the feedstock carbon and other pyrolysis products with oxygen, water, and fuel gases such as methane. The presence of oxygen, hydrogen, water vapor, carbon oxides, and other compounds in the reaction atmosphere during pyrolysis may either support or inhibit numerous reactions with carbonaceous feedstocks and with the products evolved. The distribution of weight and chemical composition of the products are also influenced by the prevailing conditions (i.e., temperature, heating rate, pressure, and residence time) and, last but by no means least, the nature of the feedstock (Speight, 2014a, 2014b, 2017).
Generally, gasification involves two distinct stages that are both feedstock and reactor dependent: (i) devolatilization to produce a semi-char which, the rate of devolatilization has passed a maximum the semi-char is converted to char by elimination of hydrogen followed by (ii) gasification of the char, which is specific to the reactor and the conditions of the reaction.
Chemically, gasification involves the thermal decomposition of the feedstock and the reaction of the feedstock carbon and other pyrolysis products with oxygen, water, and fuel gases such as methane and is represented by a sequence of simple chemical reactions (Table 14.2). However, the gasification process is often considered to involve two distinct chemical stages: (i) devolatilization of the feedstock to produce volatile matter and char, (ii) followed by char gasification, which is complex and specific to the conditions of the reaction – both processes contribute to the complex kinetics of the gasification process (Sundaresan and Amundson, 1978).
Gasification of a carbonaceous material in an atmosphere of carbon dioxide can be divided into two stages: (i) pyrolysis and (ii) gasification of the pyrolytic char. In the first stage, pyrolysis (removal of moisture content and devolatilization) occurs at a comparatively lower temperature. In the second stage, gasification of the pyrolytic char is achieved by reaction with oxygen/carbon dioxide mixtures at high temperature. In nitrogen and carbon dioxide environments from room temperature to 1000 C (1830 F), the mass loss rate o oof pyrolysis in nitrogen may be significant differently (sometime lower, depending on the feedstock) to mass loss rate in carbon dioxide, which may be due (in part) to the difference in properties of the bulk gases.
Using coal as an example ( Chapter 4), gasification in an atmosphere of oxygen/carbon dioxide environment is almost the same as gasification in an atmosphere of oxygen/nitrogen at the same oxygen concentration but this effect is a little bit delayed at high temperature. This may be due to the lower rate of diffusion of oxygen through carbon dioxide and the higher specific heat capacity of carbon dioxide. However, with an increase in the concentration of oxygen, the mass loss rate of coal also increases and, hence, shortens the burn out time of coal. The optimum value of oxygen/carbon dioxide for the reaction of oxygen with the functional groups that are present in the coal feedstock is on the order of 8% v/v.
If air is used for combustion, the product gas will have a heat content of ca. 150-300 Btu/ ft 3(depending on process design characteristics) and will contain undesirable constituents such as carbon dioxide, hydrogen sulfide, and nitrogen. The use of pure oxygen, although expensive, results in a product gas having a heat content on the order of 300 to 400 Btu/ft 3with carbon dioxide and hydrogen sulfide as byproducts (both of which can be removed from low or medium heat-content, low-Btu or medium-Btu) gas by any of several available processes (Speight, 2013, 2014a, 2017).
If a high heat-content (high-Btu) gas (900 to 1000 Btu/ft 3) is required, efforts must be made to increase the methane content of the gas. The reactions which generate methane are all exothermic and have negative values, but the reaction rates are relatively slow and catalysts may therefore be necessary to accelerate the reactions to acceptable commercial rates. Indeed, the overall reactivity of the feedstock and char may be subject to catalytic effects. It is also possible that the mineral constituents of the feedstock (such as the mineral matter in coal and biomass) may modify the reactivity by a direct catalytic effect (Davidson, 1983; Baker and Rodriguez, 1990; Mims, 1991; Martinez-Alonso and Tascon, 1991).
In the process, the feedstock undergoes three processes in its conversation to synthesis gas – the first two processes, pyrolysis and combustion, occur very rapidly. In pyrolysis, char is produced as the feedstock heats up and volatiles are released. In the combustion process, the volatile products and some of the char reacts with oxygen to produce various products (primarily carbon dioxide and carbon monoxide) and the heat required for subsequent gasification reactions. Finally, in the gasification process, the feedstock char reacts with steam to produce hydrogen (H 2) and carbon monoxide (CO).
Combustion :
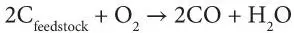
Gasification:
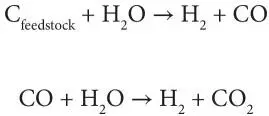
The resulting synthesis gas is approximately 63% v/v carbon monoxide, 34% v/v hydrogen, and 3% v/v carbon dioxide. At the gasifier temperature, the ash and other feedstock mineral matter liquefies and exits at the bottom of the gasifier as slag, a sand-like inert material that can be sold as a co-product to other industries (e.g., road building). The synthesis gas exits the gasifier at pressure and high temperature and must be cooled prior to the synthesis gas cleaning stage.
Although processes that use the high temperature to raise high-pressure steam are more efficient for electricity production, full-quench cooling, by which the synthesis gas is cooled by the direct injection of water, is more appropriate for hydrogen production. Full-quench cooling provides the necessary steam to facilitate the water gas shift reaction, in which carbon monoxide is converted to hydrogen and carbon dioxide in the presence of a catalyst:
Water Gas Shift Reaction:
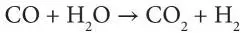
This reaction maximizes the hydrogen content of the synthesis gas, which consists primarily of hydrogen and carbon dioxide at this stage. The synthesis gas is then scrubbed of particulate matter and sulfur is removed via physical absorption (Speight, 2013, 2014a, 2017). The carbon dioxide is captured by physical absorption or a membrane and either vented or sequestered.
Читать дальше