2.2.3.7 Volumetric microsampling
Volumetric microsampling devices ( Figure 2.1) aim to collect a fixed volume of capillary blood, which can then either be made available for analysis in its entirety or can be used to provide a fixed volume of plasma for processing. These devices promise advantages over DBS related to sampling volume accuracy, simplified sample pre-treatment, and ease of automation (John et al ., 2016).
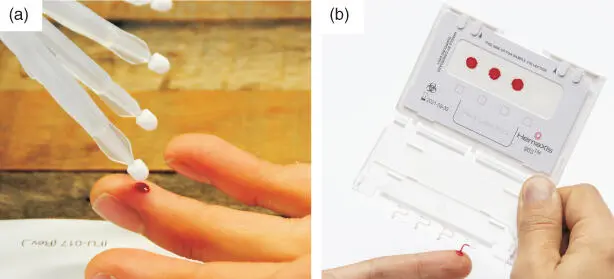
Figure 2.1 Volumetric blood microsampling devices. (a) Mitra (Neoteryx) uses an absorptive pad to collect a fixed volume of blood; (b) HemaXis DB uses a fixed volume capillary to transfer blood to a standard DBS card
Volumetric absorptive microsampling (VAMS ®) aims to absorb a fixed volume of blood or other fluid (10 or 20 μL) using the Mitra ®(Neoteryx) device (Spooner et al ., 2015; Protti et al ., 2019). Although some aspects still need to be investigated in depth (Kip et al ., 2017), VAMS ®may be a viable alternative to DBS. It simplifies sample collection and storage, and may allow sample collection in a patient's home (Kok & Fillet, 2018). The HemaXis TMDB 10 (DBS System SA) uses microfluidics to provide 10 μL whole blood from a reservoir onto a DBS card (Leuthold et al ., 2015; Verplaetse & Henion, 2016). The HemaXis TMDX promises to produce plasma or serum without the use of a centrifuge, filtration membrane, or pump and with no haemolysis. The resulting sample can be stored in dried or liquid format. The Noviplex™ Plasma Prep Card gives a fixed volume of plasma from a reservoir of whole blood (Heussner et al ., 2017).
Different urine specimens, e.g. random, early morning, end-of-shift, 24-hour (the total urine voided over a day), may be collected in the course of metabolic or other studies. In metabolic studies, it is important to note the time of the beginning and end of the collection period so that the rate of urine production can be calculated. A random urine sample is usually a midstream specimen. A preservative, for example 2 mol L –1hydrochloric acid to prevent microbial growth and to stabilize phenols, may be added. Fresh urine is yellow/yellow-green in colour, but on storage in acidic solution the colour changes to yellow/brown and even to dark brown because of the oxidation of urobilinogen to urobilin. Crystals, particularly of uric acid and calcium oxalate, may form causing turbidity.
When random, early morning, or end-of-shift specimens are collected it is common practice to relate certain analytical results to a ‘fixed’ urinary constituent such as creatinine, which is considered to be excreted at a relatively constant rate in normal subjects. However, because creatinine is derived from creatine, there are situations such as muscle wasting, excessive exercise, or in bodybuilders dosing with creatine, when this is not strictly true. Note that many clinical chemistry laboratories report creatinine in mmol L –1(1 mmol L –1= 113 mg L –1).
The concentrations of many drugs and metabolites, and of some endogenous constituents, will remain the same in acidified urine for over a week at room temperature, and for up to a month at 2–8 °C. Unacidified urine undergoes microbiological attack and many changes occur, including the complete loss of amino acids. For long term storage acidified urine can be stored at –20 °C, but it may be necessary to centrifuge the sample to remove any precipitate formed during storage prior to any analysis. Dried urine spots may also be produced in an analogous manner to dried blood spots.
This specimen encompasses vomit, gastric aspirate, and gastric lavage fluid as well as the contents of the stomach at post-mortem. The nature of this sample can be very variable and additional procedures such as homogenization followed by filtration and/or centrifugation may be required to produce a sample amenable to analysis. Sometimes tablet residues may be apparent, study of which which might help identify the drug ingested.
The analysis of faeces is rarely performed in clinical chemistry, but sometimes drug and possibly metabolite analysis may be required in pharmacokinetic and metabolism studies. Analyses may also be requested if, for example, drug leakage from ingested packets of drug is suspected. Unlike plasma, urine, and other fluid samples, faeces are not homogeneous, and thus it is often necessary to analyze the whole sample or homogenize the whole sample and prove that the fraction taken for analysis is representative of the whole. It may take more than a day after dosage before a drug or a drug metabolite appears in faeces. The stability of some drugs in meconium has been assessed (Wu et al ., 2017).
Histology specimens are usually collected into a preservative such as formalin (37 % w/v) aqueous formaldehyde solution). Such pre-treatment must be borne in mind if toxicological analyses are requested subsequently. Samples of tissue obtained post-mortem are normally kept at 2–8 °C prior to analysis.
2.3 Guidelines for sample collection for analytical toxicology
If poisoning is suspected, a 10 mL blood sample (lithium heparin or EDTA tube) should be taken from an adult (proportionally less from a young child) as soon as possible. In addition, 2 mL of blood should be collected in a fluoride/oxalate tube if ethanol is suspected. Note that tubes of this type for clinical use contain only ca . 0.1 % w/v fluoride ( Table 2.3), whereas ca . 2 % w/v fluoride (40 mg sodium fluoride per 2 mL blood) is needed to inhibit fully microbial action in such specimens. Addition of fluoride may also help protect other labile drugs such as clonazepam, cocaine, and nitrazepam from degradation. If possible, the retention of an unpreserved blood sample is also advisable.
Table 2.4 Sample requirements: general analytical toxicology
| Sample |
Notes a |
| Whole blood |
10 mL (lithium heparin or EDTA tube – use fluoride/oxalate if ethanol suspected; plastic tube if paraquat suspected; glass or plastic tube with minimal headspace if carbon monoxide or other volatiles suspected) |
| or Plasma/serum |
5 mL (send whole blood if volatiles, metals, and some other compounds suspected – see above) |
| Urine b |
20–50 mL (plain bottle, no preservative c) |
| Gastric contents d |
25–50 mL (plain bottle, no preservative) |
| Scene residues e |
As appropriate |
aSmaller volumes may often be acceptable, for example in the case of young children
bNormally the only sample that is required for substance misuse screening
cSodium fluoride (2 % w/v) should be added if ethanol is suspected and blood is not available
dIncludes vomit, gastric lavage (SWO, first sample), etc.
eTablet bottles, drinks containers, aerosol canisters, etc. – pack entirely separately from biological samples, especially if poisoning with volatiles is a possibility
Collection of urine, stomach contents, and ‘scene residues’, i.e. material such as tablet bottles or drinks containers found at the scene of an incident may be helpful ( Table 2.4). Samples of other appropriate fluids and tissues should also be collected as detailed below, especially when investigating deaths ( Table 2.5), but may not be required for analysis unless either special investigations are required or decomposition is advanced (Dinis-Oliveira et al ., 2010; Dinis-Oliveira et al ., 2017; Belsey & Flanagan, 2016). However, such samples should be retained (2–8 or –20 °C) in case they are needed. The advantages and disadvantages of various specimens are detailed in Table 2.6. There are special considerations in sample collection and storage for metals/trace elements analysis (Section 21.2).
Читать дальше













