Provided the analyte is stable, anticoagulated whole blood can be kept at room temperature or refrigerated (2–8 °C) for 2 days or so before harvesting plasma. However, leaving plasma or serum in contact with red cells can either cause changes as a result of enzymatic activity, or redistribution of an analyte between cells and plasma. Thus, in general, plasma or serum should be separated from the blood cells as soon possible. If necessary, whole blood can be stored at –20 °C or below, but freezing will lyse most cell types.
A range of anticoagulants is available for in vitro use ( Table 2.3). Sodium citrate tubes may contain 0.5 or 1 mL of an aqueous solution of anticoagulant and are unsuitable for quantitative work. Furthermore, citrate has strong buffering capacity and dilution of the sample may reduce the degree of plasma protein binding and consequently the plasma:red cell distribution of an analyte. It should be ensured that lithium heparin anticoagulant is not used if plasma lithium is to be measured (Arslan et al ., 2016). Heparin too has been known to interfere in drug analysis.
Table 2.3 Anticoagulants for in vitro use
| Anticoagulant |
Concentration (mL –1blood) |
Comment |
| Lithium heparin |
10–20 units |
General biochemistry |
| Sodium heparin |
10–20 units |
General biochemistry |
| Sodium fluoride (with either EDTA or oxalate) |
1–2 mg |
Glucose (inhibits glycolysis) |
|
6–10 mg |
General anticoagulant a |
| Sodium citrate |
3 mg |
Clotting studies – not recommended for other purposes because an aqueous solution dilutes the specimen |
| EDTA |
2 mg |
Haematology (stabilizes readily oxidized compounds) |
a ca . 2 % w/v fluoride is recommended to attempt to stabilize analytes such as cocaine ( Section 2.3) and to prevent fermentation of glucose to ethanol, for example
When whole blood is allowed to stand (15 min, room temperature) in a plain tube (no anticoagulant) a clot normally forms that will retract sufficiently to allow serum to be collected after centrifugation. For many analyses, serum is preferred to plasma because it produces less precipitate (of fibrin) on freezing and thawing.
Separation of plasma from anticoagulated whole blood normally requires centrifugation, as do many of the phase separation procedures discussed in Chapter 4. The inter-relationship of centrifuge rotor diameter, speed of centrifugation, and relative centrifugal force (g-force) is set out below ( Box 2.1). Swing-out rotors are preferred for separating liquid phases.
Box 2.1Calculating relative centrifugal force
The relative centrifugal force (RCF, g) depends upon of the speed of the centrifuge in revolutions per minute (RPM) and the effective radius of rotation, r
The radius of rotation varies along the length of the centrifuge tube
RCF may be quoted as maximum, minimum, or average
Conversion tables and nomograms for each rotor are normally supplied by the manufacturer of the centrifuge
Modern centrifuges have the facility to set the RCF directly
The RCF will be maximal at the bottom of the tube
RPM for a required RCF can be calculated from:where r is in mm
RCF from RPM is given by:
On centrifugation, anticoagulated whole blood (approximately 2000 g , 10 min; 2–8 °C if necessary) will separate into three layers: the bottom layer (normally 45 % or thereabouts by volume) consists of red cells. A thin intermediate layer of white cells and platelets called the ‘buffy coat’ is the next layer; and the upper, aqueous, straw-coloured layer is the plasma (about 50 % v/v). More plasma than serum can be separated from whole blood.
Some commercial tubes contain agents such as plastic beads or a gel that sits at the interface between the cells and the plasma to aid plasma collection. Gel separators have caused problems with some drug analyses (Berk et al ., 2006), and although reformulated gels have been shown to have little effect on clozapine and norclozapine measurement (Handley et al ., 2018), tubes containing gel separators are best avoided (Schrapp et al., 2019). Modern blood-collection tubes may contain a range of additives including surfactants, which may interfere in immunoassays, for example (Bowen & Remaley, 2014). The use of such tubes will also invalidate many trace elements analyses ( Chapter 21) and may impair analyses for solvents and other volatiles.
To collect erythrocytes, heparinized blood should be centrifuged (2000 g , 10 min), the plasma, buffy coat, and top 10 % of erythrocytes (mainly reticulocytes) removed, and the remaining erythrocytes carefully washed with isotonic phosphate-buffered saline (PBS), pH 7.4, to remove trapped plasma. The cells may be used directly or stored at –20 °C, either to cause haemolysis, or for storage. Platelets are usually isolated by the slow centrifugation (e.g. 300 g , 15 min) of anticoagulated whole blood to yield platelet-rich plasma, which is recentrifuged (2000 g , 10 min) to harvest the platelets. Other white blood cells are most commonly obtained either by centrifugation through media of appropriate density (according to manufacturers' instructions), or isolated by solid-phase antibody techniques.
Erythrocyte:plasma distribution
It is easier to measure whole blood:plasma analyte distribution than to attempt to measure the erythrocyte:plasma distribution, a measurement that requires the preparation of washed erythrocytes. Analyte is added to a portion of analyte-free heparinized whole blood and, after allowing time for equilibration and controlling the pH (the pH of blood tends to fall in vitro ), one portion of the blood is centrifuged to obtain plasma and a second portion is frozen and thawed to give haemolyzed whole blood. After measuring the analyte concentration in the plasma ( C P) and in the haemolyzed whole blood ( C B), the analyte erythrocyte concentration ( C E) can be calculated if the haematocrit, H , (the proportion of erythrocytes in the blood) is known:
(2.1) 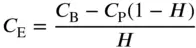
2.2.3.6 Dried blood spots
DBS are produced by the deposition of either haemolyzed whole blood, or capillary blood onto either filter paper, or dedicated paper cards. As compared with either whole blood or plasma, sample transport and storage are easier because refrigeration is not needed provided that the analyte is stable and can be recovered from the spot quantitatively (Wagner et al ., 2016; Freeman et al ., 2018). Problems are that (i) capillary blood is not venous blood, and (ii) volumetric blood collection directly from the patient onto the medium on which the blood is to be dried is extremely difficult (Sulochana et al ., 2019).
Thus, for qualitative work such as screening for inborn errors of metabolism, or for detection of substance misuse, DBS are acceptable provided that the analyte is stable in the spot, but reliable quantitative work means measuring a known volume of venous blood onto the storage medium before allowing it to dry (Section 20.3.1.1). This being said, in forensic work, measurement of the haemoglobin content of a blood spot found at a scene, for example, may serve to give a surrogate measure of the amount of blood deposited initially.
Читать дальше