In learning from the methods and sourcing of pigments from antiquity, current manufacturers of flint, amber, and green glasses and speciality coloured glasses continue to use identical materials. Metals and metallic compounds used to provide coloration and impart modifiable surface finishes include a mixture of inert and toxic substances. The overall toxicity of the glass surface is very limited, even when made with metals such as lead or cadmium, as the compounds and elements are permanently sequestered within the silicate lattice constituting the glass. Many colours of glass are so well known that they are referred to by their place of origin or the metal that they contain. The use of cobalt oxide in the glass melt is used to provide ‘cobalt blue’, which is produced by adding cobalt oxide to the glass melt. Other famous glass types include ‘jadeite or Vaseline glass’, which is a fluorescent yellow‐green glass that contains small amounts of diuranate; ‘ruby glass’ and ‘cranberry glass’, which are red glasses produced by the addition of gold or gold chloride; ‘selenium ruby glass’, which has a red tint caused by the addition of selenium oxide; and ‘Egyptian blue’, which is produced by the addition of copper. Incorporation of other colours is achieved by use of cadmium sulfide, which gives a yellow colour; cobalt oxide, which gives a violet coloration; manganese dioxide, which yields purple; and nickel oxide, which gives the melt a violet colour. More familiar colours arise from elemental sulfur, which gives yellow and browns; chromium oxide produces green; iron oxide gives green or brown; carbon gives amber or black; antimony oxide gives white; copper compounds give all the primary colours; tin compounds give white; and lead compounds give yellow coloration. In addition, manganese dioxide and sodium nitrate can be used to remove colour and therefore act as bleaching agents.
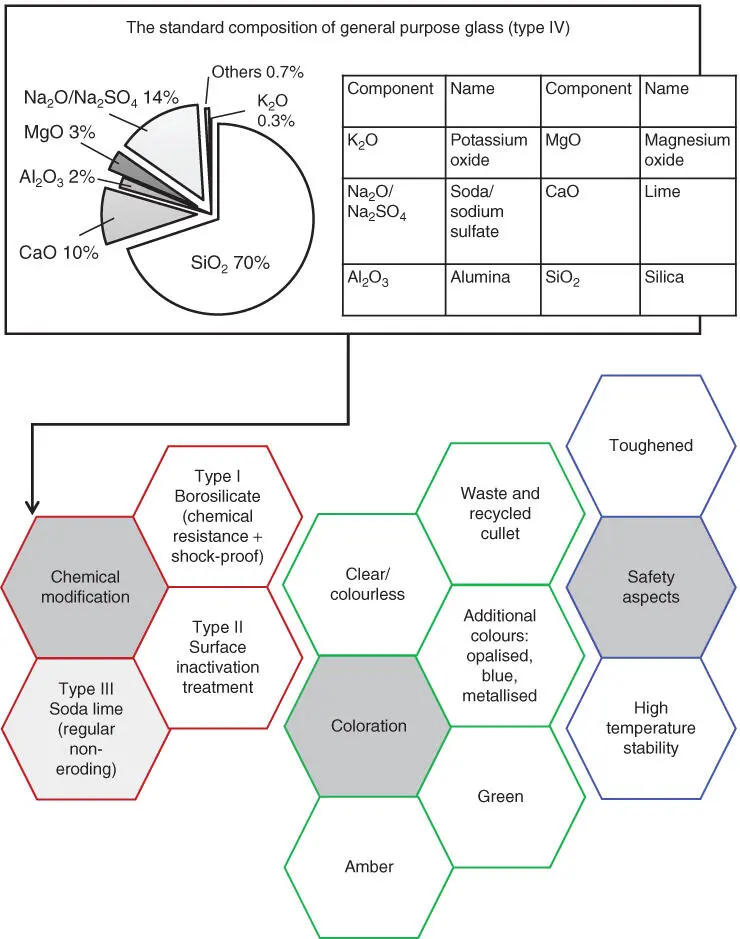
Figure 2.4 Types of glasses used in packaging applications.
Glass production is a highly energy‐consuming process in terms of the energy consumption and initial environmental impact of manufacture. Glass formed into 350 g bottles in general produces 1.06 kg of ‘greenhouse’ gases (mainly and conventionally quoted as CO 2equivalents) per kilogram of bottle material with an energy consumption of 10.5 MJ/kg, whereas polyethylene terephthalate (PET) – often considered a universal substitute for glass for both food and over‐the‐counter pharmaceutical products – produces 0.49 kg of greenhouse gases per kilogram of bottle material with an energy consumption of 0.6 MJ/kg. The implications for food and beverage containment were described as ‘one of the most intense rivalries in packaging’ by Pan Demetrakakes in the online journal Packaging Digest (USA) in September 2013 ( https://www.packagingdigest.com/beverage-packaging/material-or). In addition, the recycling of PET is much less energy consuming than that of glass. However, both materials require less energy for recycling than for original fabrication – of the order of only 74%. In general a recycled price of as little as 56% of the virgin material price is seen, with recycled glass being sold as a commodity at £20–23/tonne. Additionally, for example in the USA, approximately 3.8 times more drink bottle glass is sent for landfill disposal than PET.
There are four conventional categories of glass, which are labelled I, II, III, and IV. The classification of glass is fundamentally based around the degree of chemical attack and resistance to water‐driven hydrolysis. The extent of erosion is highly dependent on the degree of hydroxide ion release under the influence of the contact solvent but can be counteracted by inclusion of oxide compounds, such as borate, in the glassy matrix. These glass varieties range from hardened and shock‐proof borosilicate or neutral glass (type I) to general purpose soda glass (type IV), with the former used for analytical instruments and medical vials or ampoules and the latter used typically for food jars. The composition of general purpose type IV glass ( Figure 2.4) can be modified by chemical treatment. Types I–III are considered suitable in most cases for injectable or parenteral pharmaceuticals (single‐dose vials, ampoules, bottles, multi‐dose bottles) and therapeutics [12], with type I being the main variety of high‐resistivity glass used for this purpose. Type I glass vessels contain approximately 80% silica, 10% boric oxide (borate), and a small amount of sodium oxide (soda) and aluminium oxide (alumina). This gives the glass a chemical inertness and high hydrolytic resistance owing to the presence of the boric oxide. This form of glass also has the lowest coefficient of expansion and so has high thermal shock properties; however, it has a high cost per unit volume of 20–30 times that of the cheapest commercial glasses. The cost of glass varies between types with pharmaceutical‐ or pharmacopoeial‐grade type I glass being the most expensive basic form of glass. Surface treatments and lithography or printing obviously increase the commodity cost and, therefore, purchase price commensurate with the extent of mark‐up. Plain untinged or flint glass of liquid parenteral product grade is the most expensive, with a cost of approximately £0.018/g in bulk purchasing, followed by amber pharmaceutical type I glass at £0.014/g. The cheaper types of glass, generally used for food and dry medical products, are typically grade IV or superior grade III glass and scale in cost between £0.0007/g and £0.002/g, respectively. The cost is related to the required glass quality, the required transparency, the difficulty in forming the vessel (bottle, jar, ampoule, or vial), the ability to incorporate recycled cullet, and the inclusion of ‘stable’ pigments. For food‐use jars and bottles, amber and green glass are approximately 1.2–1.8 times more expensive than simple flint glass, which costs between £0.10 and £0.42 for different sizes and an array of moulded shapes for volumes ranging from 190 to 500 ml. A higher specification for exact hues in pharmaceutical‐grade tinted glass, which is not essential in food packs, lies behind the higher cost of flint over coloured glass; this is also different from food product glasses, in which recycled glass use of differing tints is commonplace.
Type I glass is suitable as a packaging material for most parenteral or non‐parenteral pharmaceutical products and is the pharmacy primary packaging standard because of its inertness and thermal stability. This type of glass possesses the highest T mvalues and so is much harder to work and shape to the desired form. The chemical robustness of borosilicate means that the glass is also ideally suited to the containment of strong acids and alkalis. Type II glass containers based on soda lime/silica glass (type III) but treated via a surface‐inactivation process to provide a contact surface that has remnant alkali ions removed is suitable for most acidic or neutral aqueous medicinal preparations, whether for parenteral or non‐parenteral use. The modification of the regular soda lime glass surface with sulfur creates a material with excellent resistance to surface hydrolytic reactions that typically occur with the ageing and weathering of glass. Modification of type III glass in this way to produce type II glass removes the sodium and calcium oxides that can be leached from water in contact with the glass surface, thereby preventing weathering and blooming from bottles. Weatherisation and ‘bloom’ formation refer to haze or visual crystalline carbonate (Na 2CO 3is the most abundant but it may also contain also CaCO 3) found on the inside of plain soda lime glass. Its appearance can alarm consumers, who mistake the clouding for possible microbial contamination and growth. The effect of weatherisation is actually minimal upon the overall quality of the glass but sodium carbonate can influence the pH of the contacting solution according to the glass formulation chemistry and solvent contact time. The hygroscopic nature of soda lime glass means that water films can easily form and accumulate on the glass surface; this happens particularly on the inside surface, where there may be water‐containing product and therefore intrinsic water vapour. Moist conditions and changes in relative humidity driven by variations in temperature, during, for example, sea freight shipping, can affect the amount of atmospheric moisture that the glass is exposed to during storage and shipment, leading to an alternating process of condensation and evaporation. Such surface adsorption can induce the dissolution of glass‐borne Na +and Ca 2+ions, which then reform as water‐dispersible carbonate crystals on the surface of the glass in the presence of carbon dioxide and as the glass surface dries. Such carbonate frosting can disappear or dissolve. Treating the surface of the glass with fluorine gas can make the surface of the glass 10 times more chemically inert and therefore less susceptible to bloom formation.
Читать дальше












