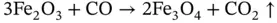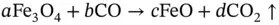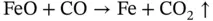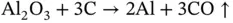Top of the furnace at lower temperature (<1000 °C), Fe(III) to Fe(III/II):
(2.1) 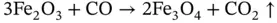
Higher temperature, Fe(III/II) to Fe(II):
(2.2) 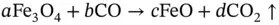
Hottest zone, Fe(II) to Fe(0):
(2.3) 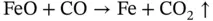
It is noteworthy that, at each step of the process to form pure metal, carbon dioxide, a so‐called ‘greenhouse gas’ and one of several causative agents of the ‘global warming’ phenomenon, is generated, as indicated by the ‘given off’ arrow (↑) in the illustrated chemical process. In the above chemical equations brackets show the oxidation state, which for iron means Fe(II) is changed to Fe(III) by the liberation of a negatively charged electron (e), thus Fe 2+→ Fe 3++ e. An oxidation state of zero for a metallic element, Fe(0), indicates a pure elemental substance (pure iron in this case).
Iron ore is first heated in a blast furnace at 2300 °C along with limestone varieties such as dolomite (CaCO 3) and coke (a source of carbon monoxide) and fuel to produce crude, high‐carbon, liquid iron that is cast into the ‘pig iron’ ingots (25 kg). Ingots can then be rolled into sheets or rods and moulded according to requirements in a hot‐press process. Further refinement of iron ingots takes place in a Bessemer (converter) furnace to produce mild steel or Linz–Donawitz‐steel‐making (basic oxygen furnace) at about 1700 °C or with a graphite electrode (EAF) at 1800 °C. The advantage of the EAF is that scrap steel can be used in the process. Aluminium ores are converted via the Bayer process from bauxite (30–60% alumina) with iron, silica, and contaminated sodium hydroxides of aluminium (Na[Al(OH) 4]) to aluminium oxide or alumina (Al 2O 3) at 180 °C under pressure. The alumina alone has a T mof greater than 2072 °C, but by being dissolved in a lower T maluminium compound (cryolite) mixed with excess fluorine in the form of fluorspar (CaF 2) a lower temperature phase transition is permitted. Purified alumina is smelted by the Hall–Héroult process via an electric furnace at 940–980 °C ( Figure 2.3). This process is based on electrolysis, where the resultant Al 2O 3is traditionally dissolved in molten cryolite (Na 3AlF 6). In modern times, because of the scarcity of the natural cryolite mineral a synthetic version (fluorite) is used, with a pure compound T mof 1012 °C but this is reduced to approximately 960 °C because of the dissolved alumina and added aluminium trifluoride (AlF 3) and by virtue of an ionic fluid being formed that is an electrically conductive medium. The electrolytic decomposition uses a consumable graphite electrode immersed in the sample with a number of concurrent reactions taking place, allowing aluminium oxide hydrolysis with associated emissions and high energy consumption [9]. The basic process in highly simplified form is:
(2.4) 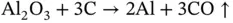
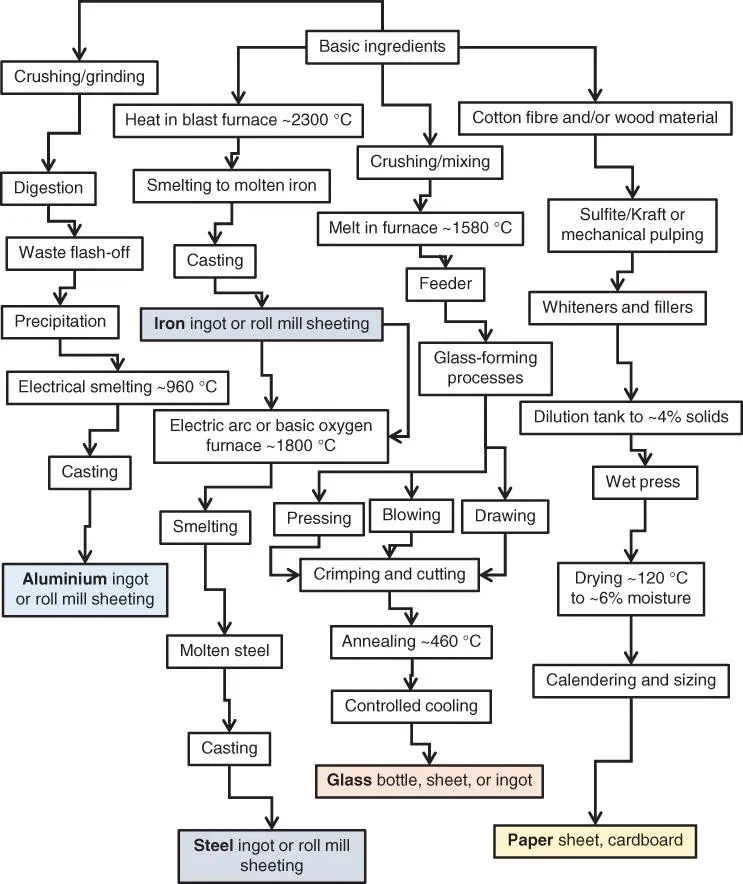
Figure 2.3 Making metal, glass, and paper packaging raw materials, where all processes end with inspection and testing.
This process involves the generation of several electrons at the cathode and their absorption at the anode. Importantly, carbon dioxide is produced in the presence of oxygen and carbon monoxide ( Eq. 2.4). These are gases of environmental significance along with the noxious fluorine waste compounds (e.g. hydrogen fluoride and chlorofluorocarbons [CFCs]), with a need for safety and that on mass production leave a weighty carbon footprint [10]. These compounds contribute to global warming (CFCs, along with methane and carbon dioxide), as the perhaps most infamous of the ‘greenhouse’ gases, topped only by the relatively rare coolant gas sulfur hexafluoride (SF 6). The graphitic cathode (negative electrode) and positive electrode (anode) are made of elemental carbon. As part of the process liquid aluminium metal forms at the cathodic electrode and sinks to the bottom of the tank, where it is siphoned off, because of its higher density even at this high temperature. Liquid aluminium is cast into ingots of approximately 20 kg, which is double the ingot size of metals such as copper.
2.3.2 Forming and Sheet‐Making
Glass (plain glass or soda glass) is manufactured at 1580 °C in a furnace with a mixture of cullet (broken glass), fine sand, lime (CaO), and soda (NaO). The molten glass is then pressed, drawn, blown, and shaped into the desired form ( Figure 2.3). After forming into bottles the glass is crimped for effective container closure. The bottles are annealed at 460 °C to aid removal of internal stresses, then allowed to cool without defect, fissure, or crack formation. Coloured glasses are blended to give the same continuous output of glass.
Paper is a highly recyclable commodity like glass, steel, and aluminium. The Kraft and sulfite/sulfate processes for making paper are the most common forms of paper fibre manufacture. Paper is manufactured from Kraft‐, mechanical‐, or sulfite‐treated wood chips and then formulated with fillers and whiteners such as kaolin and gums, e.g. poly(vinyl alcohol). After dilution to about 4% solids by weight and formation by the Fourdrinier process (see Figure 5.2) on a mesh, from the deposited aqueous slurry, the samples are wet pressed. Following on from basic sheet‐making the ‘wet’ mass is dried at 120 °C to a final moisture content of approximately 6% w/w ( Figure 2.3). Calendering, the process of making a uniform thickness by pressured successive rolling follows (at 20 kPa–40 MPa), terminated by sizing. Rolls of finely graded 0.5 mm thickness processed paper of 2.5 m spool width, 2.5 m spool diameter, and a supporting roll core of 0.25 m can weigh 8 tonnes. The crude paper is then ready for the final step, which is grading for application depending on its coarseness and degree of refining.
Product quality tests form an essential part of maintaining the consistency of the raw materials produced. For metals this would be reflected in the chemical distribution and ratios of minor elements or impurities, such as copper, arsenic, or sulfur [11]. Metal quality is also routinely evaluated through mechanical indices such as tensile strength and hardness as well as sheen and lustre. Glass quality would be defined by uniform transparency and shading and freedom from wall variation thickness, micro‐fissures (air‐tightness), bubbles, blisters, cracks, or erroneous moulding. Paper quality is usually estimated in terms of tear and crease resistance.
Glass‐blowing is a craft that was demonstrated in the first glass manufactured in 3500 BCE, which comes from either Syria, Mesopotamia, or Egypt. During the late Bronze age, c. 1550–1200 BCE, there was significant growth in the semi‐industrial production of blown glass in Egypt. In the fifteenth century BC in Crete, Egypt, and the western parts of Asia (Near East) improvement in fabrication and technique followed with the very first formal glass‐making manual, which was created in 650 BC. Expertise in glazing, surface texturisation, and material mixing was honed in the third century BC in India, and by the first century BC Roman glass had started to become famed for its clarity and purity by virtue of using high‐grade white sand. In the seventh to eighth centuries patterned Venetian glass became known for its excellence and was highly sought after across the globe. The masters of glass‐making in the past were considered to be the Phoenicians and Romans and significant aspects of their accrued expertise are still used in modern glass manufacture. Simultaneously, in the eighth century, coloured glass innovations and mould‐blowing or mould‐press‐forming mastery developed in the Sasanian Empire, based in Persia (Iran, Iraq, Syria, Egypt, Yemen, Palestine, and Pakistan), led to the invention of fabrication instruments still currently in use. Three centuries later mirrored glass was first fabricated in Moorish Spain. Stained glass, fabricated using thermally stable particulate metals and metal oxide salts, was first used in a civil engineering context in the twelfth century. In 1674 George Ravenscroft created clear lead crystal known as ‘flint’ glass in England, and by 1830–1850 the so‐called Stoddard utility bottle could be found routinely across the USA. This was driven by an increased demand for glass containers and a means of high‐output manufacture blow‐mould technology that was first developed in 1910 and is still used today. Glass is made from sand (∼59%), soda ash–sodium carbonate (∼18.5%), lime–limestone (∼17%), and 4–6% other substances (Fe, Cr, Co, Se, Na 2SO 4, calumite, nepheline syenite). Some glass makes use of cullet (as much as 10%) or ground recycled glass. Many speciality varieties of packaging glass exist in the form of clear, amber, green, blue, white, opalescent, or metallised forms. Preparation of glass materials involves sourcing appropriate materials of the correct grade, melting and conditioning at 1400–1600 °C, fashioning into appropriate forms ( Figure 2.4), annealing, inspection for defects, and ultimately dispatch.
Читать дальше