The Actinobacteria , including important pathogens such as Mycobacterium tuberculosis , utilize a proteasome structure similar to that used in eukaryotes to mediate degradation of specific protein targets. This structure is barrel-shaped and carries out both ATP-dependent and ATP-independent proteolysis. Eukaryotes use a specific protein tag called ubiquitination to direct protein substrates to the proteasome; in Actinobacteria , this is replaced by a different protein modification called pupylation (see Becker and Darwin, Suggested Reading).
About one-fifth of the proteins made in a bacterium do not remain in the cytoplasm and instead are transported or exported into or through the surrounding membranes. The terminology is often used loosely, but we refer to proteins that leave the cytoplasm as being transported. The process of transferring them through one or both membranes is secretion. If they are transferred through both membranes to the exterior of the cell, they are exported. Correspondingly, proteins that remain in either the inner or outer membrane are inner membrane proteinsor outer membrane proteins, while those that remain in the periplasmic space are periplasmic proteins. Proteins that are passed all the way out of the cell into the surrounding environment are exported proteins.
By far the largest group of proteins that are transported from the cytoplasm are destined for the inner membrane. Inner membrane proteins often extend through the membrane a number of times and have some stretches that are in the periplasm and other stretches that are in the cytoplasm. The stretches that traverse the membrane have mostly uncharged, nonpolar (hydrophobic [see the inner cover]) amino acids, which make them more soluble in the membranes. A stretch of about 20 mostly hydrophobic amino acids is long enough to extend from one side of the bipolar lipid membrane to the other, and such stretches in proteins are called the transmembrane domains. The less hydrophobic stretches between them are called the cytoplasmic domainsor periplasmic domains, depending on whether they extend into the cytoplasm on one side of the membrane or into the periplasm on the other side. Proteins with domains on both sides of the membrane are called transmembrane proteinsand are very important, because they allow communication from outside the cell to the cytoplasm. Some transmemembrane proteins that play such a communicating role are discussed in chapter 12.
Transported proteins usually contain many amino acids that are either polar or charged (basic or acidic), which makes it difficult for them to pass through the membranes. They must be helped in their translocation through the membrane by other specialized proteins. Some of these proteins form a channel in the membrane. Some transported proteins make their own dedicated channel, but most use the more general channel called the translocase, so named because its function is to translocate proteins.
A current picture of the structure of the translocase that helps proteins pass through the inner membrane, as well as how it works, is outlined in Figure 2.38. We can predict some of the features this channel must have. It must have a relatively hydrophilic inner channel through which charged and polar amino acids can pass. It also must normally be closed and should open only when a protein is passing through it; otherwise, other proteins and small molecules would leak in and out of the cell through the channel. Even the leakage of molecules as small as protons cannot be tolerated, because it would destroy the proton motive force. The channel is made up of one each of three proteins, SecY, SecE, and SecG, and is therefore called the SecYEG channelor SecYEG translocase. These three proteins form a heterotrimer made up of one each of the three different polypeptides. The SecY protein is by far the largest of the three proteins and forms the major part of the channel, while the other two proteins play more ancillary, albeit important, roles. One heterotrimer can form a large enough channel to let an unfolded protein through (see van den Berg et al., Suggested Reading), but it seems likely that more than one of these heterotrimers is involved.
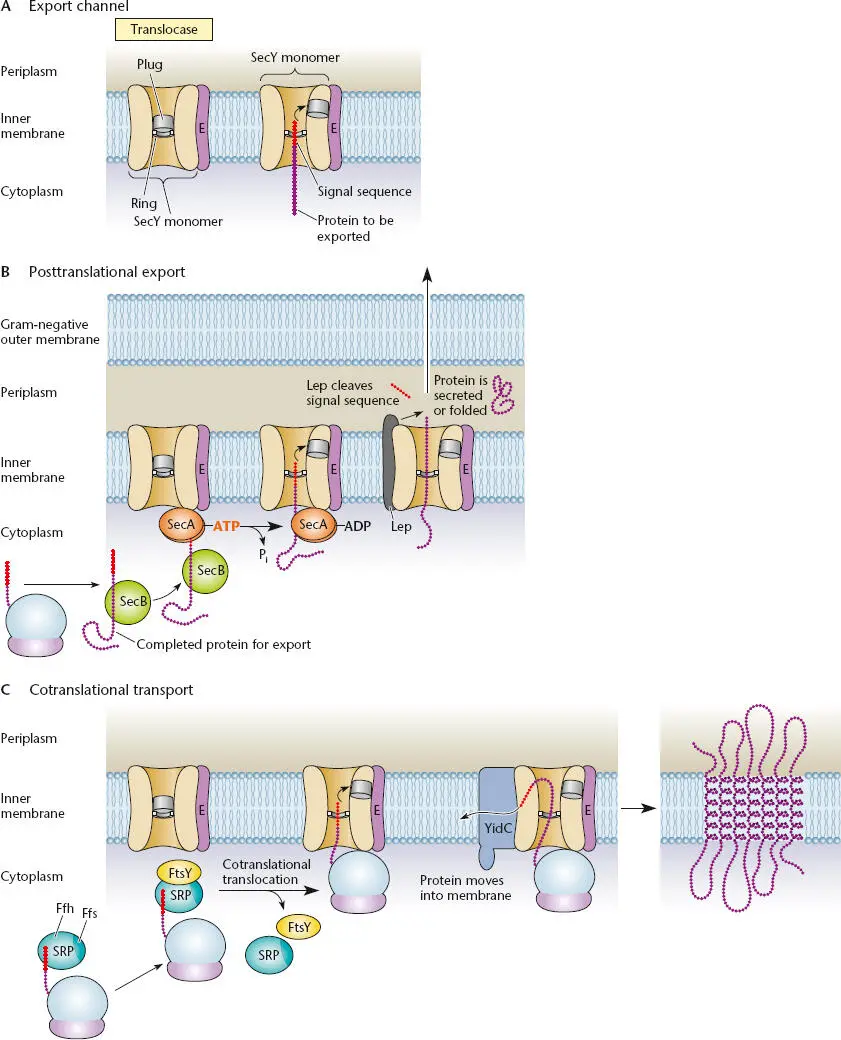
Figure 2.38 Protein transport systems. (A)Cutaway view of the secretion sec channel. SecY, SecE, and SecG (not shown) form the translocase. SecY forms the channel, ring, and plug. The signal sequence of the transported protein moves the plug toward SecE. (B)Posttranslational secretion by the SecB-SecA system. SecB keeps the protein unfolded until it binds to SecA, which interacts with SecY. The signal sequence is removed, in this case by Lep protease. The exported protein is folded in the periplasm or may be secreted across the outer membrane by one of the dedicated secretion systems. (C)Cotranslational transport by the signal recognition particle (SRP) system. SRP binds to the first transmembrane domain as it emerges from the ribosome and then binds to the FtsY docking protein, bringing the ribosome to interact with SecY. The protein is translated, driving it into the SecYEG channel. The transmembrane domains of the protein somehow escape through the side of the channel into the membrane, in some cases with the help of the YidC protein, as shown.
Besides forming the major part of the channel, a region of the SecY protein forms a hydrophobic “plug,” which opens only when a protein is passing through ( Figure 2.38). The binding of a signal sequence(see below) in a protein to be transported causes the plug to move over toward SecE on the side of the channel, opening the channel. As a result, only proteins that have a bona fide signal sequence can be translocated.
As mentioned above, the defining feature of proteins that are to be transported into the inner membrane or beyond by the SecYEG channel is the presence at their N termini of a signal sequence. The nature and fate of this signal sequence depend upon the ultimate destination of the transported protein. For proteins that are to be transported through the inner membrane into the periplasm and beyond, the signal sequence is approximately 20 amino acids long and consists of a basic region at the N terminus, followed by a mostly hydrophobic region and then a region with some polar amino acids. In contrast, most proteins whose final destination is the inner membrane merely use their first N-terminal transmembrane domain as a signal sequence.
If the protein is to be secreted through the membrane, the signal sequence is removed by a protease as the protein passes through the SecYEG channel ( Figure 2.38B). The most prevalent of the proteases that clip off signal sequences in E. coli is the Lep protease(for leader peptide protease), but there is at least one other, more specialized protease called LspA, which removes the leader sequence from some lipoproteins destined for the outer membrane. Proteins that are destined to be transported beyond the inner membrane but have just been synthesized and so still retain their signal sequences are called presecretory proteins. When the short signal sequence is removed in the SecYEG channel, the presecretory protein becomes somewhat shorter before it reaches its final destination in the periplasm or the outer membrane or outside the cell.
Читать дальше












