TRIGGER FACTOR AND OTHER CHAPERONES
Given the prevalence and central role of DnaK in the cell, it came as a surprise that E. coli mutants that lack DnaK still multiply, albeit slowly. In fact, the only reason they are sick at all is because they are making too many copies of the other heat shock proteins, since DnaK also regulates the heat shock response (see chapter 12). One reason why cells lacking DnaK are not dead is that other chaperones can substitute for it. One of these is trigger factor. This type of chaperone has so far been found only in bacteria, and much less is known about it. It binds close to the exit pore of the ribosome and helps proteins fold as they emerge from the ribosome. It is also a prolyl isomerase. Of all the amino acids, only proline has an asymmetric carbon, which allows it to exist in two isomers. Trigger factor can convert the prolines in a protein from one isomer to the other. There are many other examples of chaperones that act as prolyl isomerases.
Another set of chaperones, including ClpA, ClpB, and ClpX, form cylinders and unfold misfolded proteins by sucking them through the cylinder. This takes energy, which is derived from cleavage of ATP. Some of them, including ClpA and ClpX, can also feed the unfolded proteins directly into an associated protease called ClpP, which degrades the unfolded protein. Association with ClpP switches the function of ClpA and ClpX from protein folding to protein degradation. ClpB, another cylindrical chaperone, does not associate with a protease but seems to cooperate with the small heat shock proteins IbpA and IbpB to help redissolve precipitated proteins so that they can be refolded by DnaK (see Mogk et al., Suggested Reading).
In addition to the relatively simple protein chaperones, cells contain much larger structures that help proteins fold. These large structures are called chaperonins, and they exist in all forms of life, including the archaea and eukaryotes. They are composed of two large cylinders with hollow chambers held together back to back with openings at their ends ( Figure 2.37). They help fold a misfolded protein by taking it up into one of the chambers. A cap, called a cochaperonin, is then put on the chamber, and the protein folds within the more hospitable environment of the chamber. A more detailed model for what happens in the chamber and how this helps a protein fold is suggested by the structure (see Wang and Boisvert, Suggested Reading). When the misfolded protein is first taken up, the lining of the chamber consists of mostly hydrophobic amino acids that bind to the exposed hydrophobic regions of the misfolded protein. When the cochaperonin cap is put onto this chamber (the cis -chamber) and the bound ATP is cleaved to ADP, the lining may switch to being mostly hydrophilic amino acids, driving the more hydrophobic regions of the misfolded protein to the interior of the protein, where they usually reside in the folded protein. Binding of ATP and an unfolded protein to the other chamber (the trans-chamber ) causes the cap to come off the cis -chamber, releasing the folded protein and preparing the other chamber to take up the misfolded protein and take its turn being the cis -chamber. This process takes a lot of energy, and a number of ATP molecules (one for each subunit of one chamber of the chaperonin, which is seven in E. coli [see below]) are cleaved to ADP in each cycle. It is a mystery why chaperonins have two chambers and why the folding has to alternate between the two chambers. Chaperonins composed of only one chamber function more poorly, although they might retain some of their activity (see Sun et al., Suggested Reading). The communication to one chamber that ATP and unfolded protein are bound to the opposite chamber is one of the most remarkable examples of allosterismknown, especially considering that the chambers, while touching back to back, are composed of separate polypeptides.
The first chaperonin was discovered in E. coli , where it was named GroEL because it helps assemble the E protein of phage λ into the phage head. The GroEL chaperonin consists of 14 identical polypeptides (7 making up each cylinder) of 60 kDa. Its cochaperonin cap is called GroES, which is also made up of 7 subunits, each 10 kDa in size. Unlike DnaK and the other chaperones, GroEL and GroES are required for E. coli growth, even at lower temperatures. The GroEL chaperonin is known to be required for folding of some essential proteins, which explains why GroEL is essential. The chaperonins come in two general types called the group I and group II chaperonins. The group I chaperonins, related to GroEL, are composed of 60-kDa subunits and are found in all bacteria and in the mitochondria and chloroplasts of eukaryotes; this makes sense, since these organelles are derived from bacteria (see the introductory chapter). These chaperonins and their cochaperonins are induced by heat shock and other stresses, so they are called the Hsp60 proteins and Hsp10 proteins for heat shock 60-kDa and 10-kDa proteins (see Bukau and Horwich, Suggested Reading). The group II chaperonins are found in the archaea and in the cytoplasm of eukaryotes. They have very little amino acid sequence in common with the group I chaperonins and are not composed of identical subunits (i.e., they are mixed multimers) and often have eight or more polypeptide subunits per cylinder. Furthermore, if they have a cochaperonin cap, it might be attached to the opening of the chamber rather than being detachable, like GroES. They may also be more dedicated and fold only a small subset of proteins, including actin in the eukaryotic cytoplasm. Nevertheless, the two types form similar cylindrical structures and presumably use a similar two-stroke mechanism to help fold proteins. Note that this is yet another example where the archaea and eukaryotes are similar to each other and different from the bacteria (see the introductory chapter).
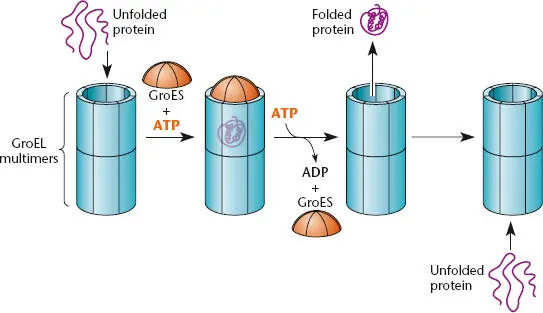
Figure 2.37 Chaperonins. The GroEL (Hsp60)-type chaperonin multimers form two connected cylinders. A denatured or unfolded protein enters the chamber in one of the cylinders, and the chamber is capped by the cochaperonin GroES (Hsp10). The denatured protein can then be helped to fold in the chamber and is released. Entry of a second unfolded protein into the second cylinder helps to trigger release of the folded protein.
As noted above, protein folding can be coupled to protein degradation through the association of chaperones such as ClpA and ClpX with proteases such as ClpP. Complexes such as ClpAP and ClpXP are members of the family of ATP-dependent proteases, which use cleavage of ATP to provide the energy for protein unfolding and proteolysis (see Baker and Sauer, Suggested Reading). These enzymatic machineries are important not only for the destruction of misfolded and denatured proteins, but also for regulated proteolysis to target specific substrate proteins under specific conditions. Their activity can be directed by the presence of a specific degradation tag, a short protein sequence that increases affinity for a specific protease; these tags are similar to the sequence added by tmRNA to target incomplete proteins for degradation (see above). This tag may be present all the time within the target protein, but it becomes available for recognition only under certain conditions, which allows the target protein to be stable under some conditions and unstable under other conditions. Proteolysis can also be controlled by adaptor proteins that deliver specific target proteins to specific proteases. Regulated proteolysis is discussed as a mechanism for gene regulation in chapters 11and 12.
Читать дальше












