Jane Flint - Principles of Virology
Здесь есть возможность читать онлайн «Jane Flint - Principles of Virology» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Principles of Virology
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:3 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 60
- 1
- 2
- 3
- 4
- 5
Principles of Virology: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Principles of Virology»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
Volume I: Molecular Biology
Volume II: Pathogenesis and Control
Principles of Virology, Fifth Edition
Principles of Virology — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Principles of Virology», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
A critical regulator of the receptor-induced structural transitions of poliovirus particles appears to be a hydrophobic tunnel located below the surface of each structural unit ( Fig. 5.22). The tunnel opens at the base of the canyon and extends toward the 5-fold axis of symmetry. In poliovirus type 1, each tunnel is occupied by a molecule of sphingosine. Similar lipids have been observed in the capsids of other picornaviruses. Because of the symmetry of the capsid, each virus particle may contain up to 60 lipid molecules. These lipids are thought to contribute to the stability of the native virus particle by locking the capsid in a stable conformation. Consequently, removal of the lipid is probably necessary to endow the particle with sufficient flexibility to permit the RNA to leave the protein shell.
The viral genome is released from the endosome, and it is usually assumed that the 5′ end of (+) strand RNAs is the first to leave the capsid, to allow immediate initiation of translation by ribosomes. This assumption is incorrect for rhinovirus type 2: exit of viral RNA starts from the 3′ end. This directionality is a consequence of how the viral RNA is packaged in the virus particle, with the 3′ end near the location of pore formation in the altered particle. Whether such directionality is a general feature of nonenveloped (+) strand RNA viruses is unknown.
Similar to picornaviruses, another family of nonenveloped (+) strand RNA viruses, caliciviruses, also form pores in the endosomal membrane. Binding to the receptor triggers conformational changes in the viral capsid, and following endocytosis, the capsid protein VP2 forms a large portal at the 3-fold axis of symmetry. This portal would allow delivery of the RNA genome to the cytoplasm.
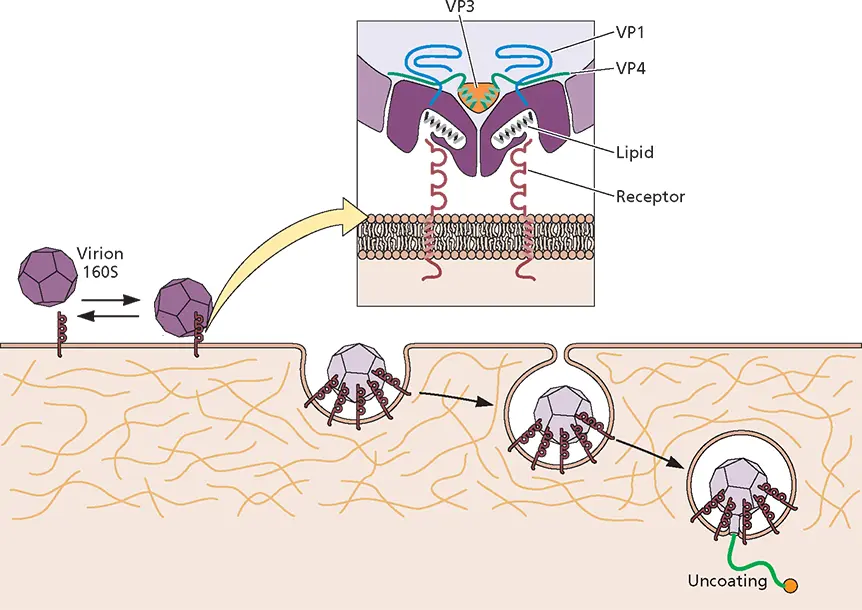
Figure 5.22 Model for poliovirus entry into cells. The native virus particle (160S) binds to its cell receptor, CD155, and undergoes a receptor-mediated conformational transition resulting in the formation of altered (A) particles. Shortly after endocytosis and close to the plasma membrane, the viral RNA leaves the capsid. A long, umbilical connector is formed between the particles and the endosomal membrane that allows the RNA to escape. (Inset) Cross-section of poliovirus particle bound to CD155. Capsid pockets are occupied by lipids that may contribute to capsid stability.
Disrupting the Lysosomal Membrane
Most virus particles that enter cells by receptor-mediated endocytosis leave the pathway before the vesicles reach the lysosomal compartment. This departure is not surprising, for lysosomescontain proteases and nucleases that would degrade virus particles. However, these enzymes play an important role during the uncoating of members of the Reoviridae .
Orthoreoviruses are naked icosahedral viruses containing a double-stranded RNA genome of 10 segments. The viral capsid is a double-shelled structure assembled from eight different proteins. These virus particles bind to cell receptors via protein σ1 and are internalized into cells by endocytosis ( Fig. 5.23). The intact virus particle comprises two concentric, icosahedrally organized protein capsids. The outer capsid is made up largely of σ3 and μ1. The dense core shell is formed mainly by λ1 and σ2.
Infection of cells by reoviruses is sensitive to bafilomycin A1, an inhibitor of the endosomal proton pump, indicating that acidification is required for entry. Disassembly occurs in multiple steps while the virus particle travels within endosomes to the lysosome ( Fig. 5.23A). The process is initiated with the acid-induced proteolysis that releases the 600 σ3 subunits of the capsid. The μ1 protein changes from a compact form to an extended flexible fiber, producing an infectious subviral particle (ISVP). The μ1 protein undergoes significant conformational changes and is cleaved at three sites, one of which releases the myristoylated N terminus, μ1N, which can insert into membranes ( Fig. 5.23B). Both μ1N and μ1C are required for membrane penetration. Isolated ISVPs cause cell membranes to become permeable to toxins and produce pores in artificial membranes. These can also initiate an infection by penetrating the plasma membrane, entering the cytoplasm directly. Their infectivity is not sensitive to bafilomycin A1, further supporting the idea that these particles are primed for membrane entry and do not require further acidification for this process.
The core particles generated from infectious subviral particles after penetration into the cytoplasm adopt a third morphology and carry out viral mRNA synthesis. The core is produced by the release of 12 σ1 fibers and 600 μ1 subunits. In the transition from ISVP to core, domains of λ2 rotate upward and outward to form a turret-like structure ( Fig. 5.23A).
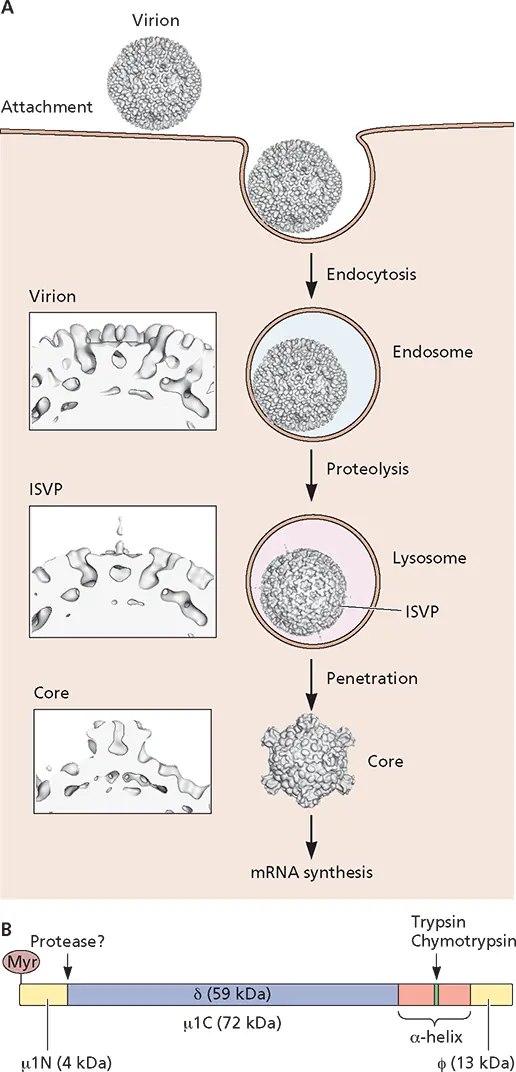
Figure 5.23 Entry of reovirus into cells. (A)The different stages in cell entry of reovirus. After the attachment of σ1 protein to the cell receptor, the virus particle enters the cell by clathrin-mediated endocytosis. Proteolysis in the late endosome produces the infectious subviral particle (ISVP). The viral μ1, a myristoylated protein, is located at the surface of these particles and interacts with membranes. Consequently, subviral particles penetrate the lysosomal membrane and escape into the cytosol. (Insets) Close-up views of the emerging turret-like structure as the virus progresses through the ISVP and core stages. This structure may facilitate the entry of nucleotides into the core and the exit of newly synthesized viral mRNAs. (B)Schematic of the μ1 protein, showing locations of myristate and the protease cleavage sites flanked by the amphipathic α-helices. Virus images based on studies performed with mammalian reovirus type I Lang, reprinted from Dryden KA et al. 1993. J Cell Biol 122:1023–1041, with permission. Courtesy of Norm Olson and Tim Baker, Purdue University.
Import of Viral Genomes into the Nucleus
The reproduction of most DNA viruses, and some RNA viruses including retroviruses and influenza viruses, begins in the cell nucleus. The genomes of these viruses must therefore be imported from the cytoplasm. One way to accomplish this movement is via the cellular pathway for protein import into the nucleus. An alternative, observed in cells infected by some retroviruses, is to enter the nucleus after the nuclear envelope breaks down during cell division.
The Nuclear Pore Complex
The nuclear envelope is composed of two typical lipid bilayers separated by a luminal space. Like all other cellular membranes, it is impermeable to macromolecules such as proteins. However, the nuclear pore complexes that stud the nuclear envelopes of all eukaryotic cells provide aqueous channels that span both the inner and outer nuclear membranes for exchange of small molecules, macromolecules, and macromolecular assemblies between nuclear and cytoplasmic compartments. Numerous experimental techniques, including direct visualization of gold particles attached to proteins or RNA molecules as they are transported, have established that nuclear proteins enter and RNA molecules exit the nucleus by transport through the nuclear pore complex. The functions of the nuclear pore complex in these processes are not completely understood, not least because this important cellular machine is large (molecular mass, approximately 125 × 10 6kDa in vertebrates), built from many different proteins, architecturally elaborate, and dynamic ( Fig. 5.24).
Читать дальшеИнтервал:
Закладка:
Похожие книги на «Principles of Virology»
Представляем Вашему вниманию похожие книги на «Principles of Virology» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Principles of Virology» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.











