Jane Flint - Principles of Virology
Здесь есть возможность читать онлайн «Jane Flint - Principles of Virology» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Principles of Virology
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:3 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 60
- 1
- 2
- 3
- 4
- 5
Principles of Virology: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Principles of Virology»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
Volume I: Molecular Biology
Volume II: Pathogenesis and Control
Principles of Virology, Fifth Edition
Principles of Virology — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Principles of Virology», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
As might be anticipated, elucidation of the structures of virus particles and individual structural proteins has illuminated the mechanisms of both assembly of viral nanomachines in the final stages of an infectious cycle and their entry into a new host cell. High-resolution structural information can also facilitate identification of targets for antiviral drugs, as well as the design of such drugs (Volume II, Chapter 8), and provide insights into the dynamic interplay between important viral pathogens and host adaptive immune responses (Volume II, Chapter 4). As we shall see, cataloguing of virus architecture has also revealed completely unanticipated relationships among viruses of different families that infect evolutionarily divergent hosts, and has suggested new principles of virus classification.
Nomenclature
Virus architecture is described in terms of structural unitsof increasing complexity, from the smallest biochemical unit (the polypeptide chain) to the infectious particle (or virion). These terms, which are used throughout this text, are defined in Table 4.2. Although virus particles are ordered assemblies of macromolecules exquisitely suited for protection and delivery of viral genomes, they are constructed according to the general principles of biochemistry and protein structure.
Methods for Studying Virus Structure
Electron microscopy is the most widely used method for the examination of structure and morphology of virus particles. This technique, which has been applied to viruses since the 1940s, traditionally relied on staining of purified virus particles (or of sections of infected cells) with an electron-dense material. It can yield quite detailed and often beautiful images ( Fig. 1.9; see the Appendix) and provided the first rational basis for classification of viruses.
Table 4.2 Nomenclature used in description of virus structure
| Term | Synonym | Definition |
|---|---|---|
| Subunit (protein subunit) | Single, folded polypeptide chain | |
| Structural unit | Asymmetric unit | Unit from which capsids or nucleocapsids are built; may comprise one protein subunit or multiple, different protein subunits |
| Capsid | Coat | The protein shell surrounding the nucleic acid genome |
| Nucleocapsid | Core | The nucleic acid-protein assembly packaged within the virion; used when this assembly is a discrete substructure of a particle |
| Envelope | Viral membrane | The host cell-derived lipid bilayer carrying viral glycoproteins |
| Virion | The infectious virus particle |
The greatest contrast between virus particle and stain (negative contrast) occurs where portions of the folded protein chain protrude from the surface. Consequently, surface knobs or projections, termed morphological units, are the main features identified by this method. However, because these surface features are often formed by multiple proteins, their organization does not necessarily correspond to that of the individual proteins that make up the capsid shell. Even when structure is well preserved and a high degree of contrast can be achieved, the minimal size of an object that can be distinguished by classical electron microscopy, its resolution, is limited to 50 to 75 Å. Th is resolution is far too poor to permit molecular interpretation: for example, the diameter of an α-helix in a protein is on the or der of 10 Å. Cryo-electron microscopy (cryo-EM), in which samples are rapidly frozen and examined at very low temperatures in a hydrated, vitrified (noncrystalline, glass-like) state, preserves native structure. Because samples are not stained, this technique allows direct visualization of the contrast inherent to the virus particle, and it also enables higher resolution.
The symmetry of most virus particles facilitates analysis of individual images obtained by cryo-EM for reconstruction of two- or three-dimensional structure ( Box 4.1). This approach can be complemented by cryo-electron tomography, in which two-dimensional images are recorded as the vitrified sample is tilted at different angles to the electron beam and subsequently combined into a three-dimensional density map ( Fig. 4.3). These methods have been greatly improved since their introduction, to near atomic-level resolution, and are now standard tools of structural biology. Their application has provided unprecedented views of virus particles not amenable to other methods of structural analysis, for example, alphaviruses ( Fig. 4.23) and herpesviruses ( Fig. 4.27).
The first descriptions of the molecular interactions that dictate the structure of virus particles were obtained by X-ray crystallography ( Fig. 4.4) (see the interview with Dr. Michael Rossmann: http://bit.ly/Virology_Rossmann). A plant virus (tobacco mosaic virus) was the first to be crystallized, and the first high-resolution virus structure determined was that of tomato bushy stunt virus. Since this feat was accomplished in 1978, high-resolution structures of increasingly large animal viruses have been determined, placing our understanding of the principles of capsid architecture on a firm foundation.
BOX 4.1
METHODS
The development of cryo-electron microscopy, a revolution in structural biology
Cryo-EM has now revealed near-atomic resolution of not only symmetric virus particles, some very large, but also asymmetric and dynamic cellular machines built from many components, such as transcription complexes and the spliceosome. The foundations for this revolutionary method of structural biology were laid in the 1970s and 1980s by Jacques Dubochet, Joachim Frank, and Richard Henderson, whose contributions were recognized by the 2017 Nobel Prize in Chemistry.
Henderson was the first to use electron microscopy to investigate the structure of a protein (bacteriorhodopsin in a cell membrane), and obtained a low-resolution model. The development by Frank of algorithms for the sorting of randomly oriented molecules into related groups for averaging improved the resolution of two-dimensional images and facilitated their transformation into three-dimensional structural models ( Fig. 4.3). Further increases in resolution were achieved when Dubochet perfected methods for vitrification of samples to produce much sharper images.
Near-atomic resolution, which is now quite routine, was attained with additional refinements, including the use of direct electron detectors (rather than film or CCD cameras) to capture images and increasingly sophisticated data processing software.
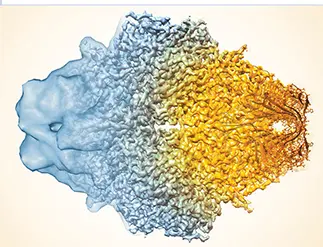
The composite image of cryo-EM reconstructions of the enzyme β-galactosidase dramatizes the great improvement in resolution, from the 10 to 20 Å typical a decade ago to, in this case, 2.2 Å (left to right).Courtesy of Sriram Subramanian, National Cancer Institute.
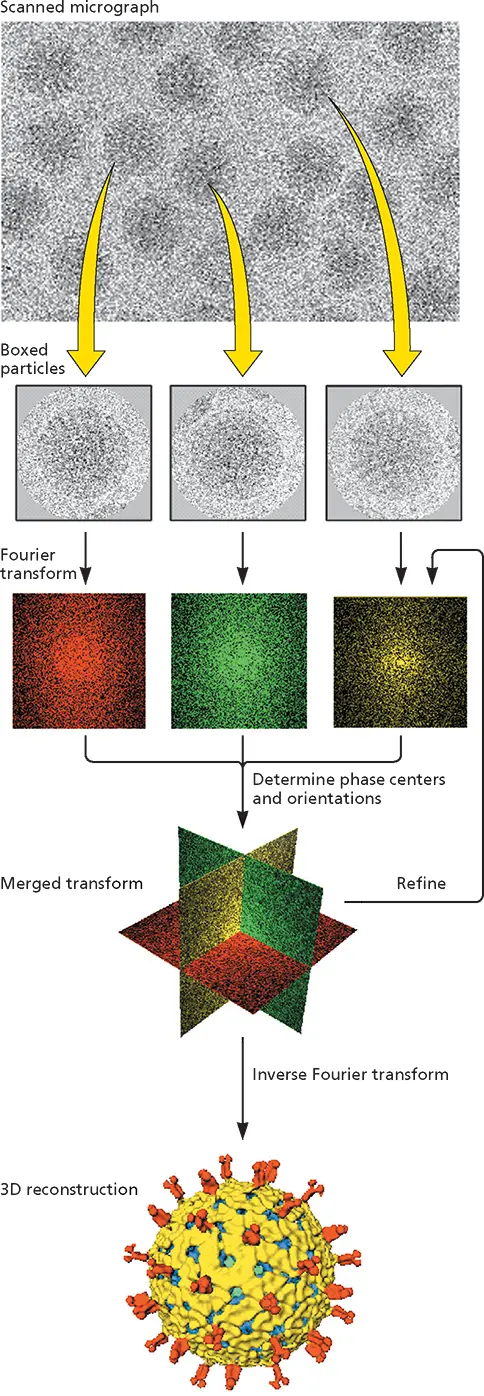
Figure 4.3 Cryo-EM and image reconstruction illustrated with rotavirus.
Concentrated preparations of purified virus particles are prepared for cryo-electron microscopy by rapid freezing on an electron microscope grid so that a glasslike, noncrystalline water layer is produced. This procedure avoids sample damage that can be caused by crystallization of the water or by chemical modification or dehydration during conventional negative-contrast electron microscopy. The sample is maintained at or below −160°C during all subsequent operations. Fields containing sufficient numbers of vitrified virus particles are identified by transmission electron microscopy at low magnification (to minimize sample damage from the electron beam) and photographed at high resolution (top).
Читать дальшеИнтервал:
Закладка:
Похожие книги на «Principles of Virology»
Представляем Вашему вниманию похожие книги на «Principles of Virology» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Principles of Virology» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.











