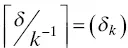1 ...6 7 8 10 11 12 ...27 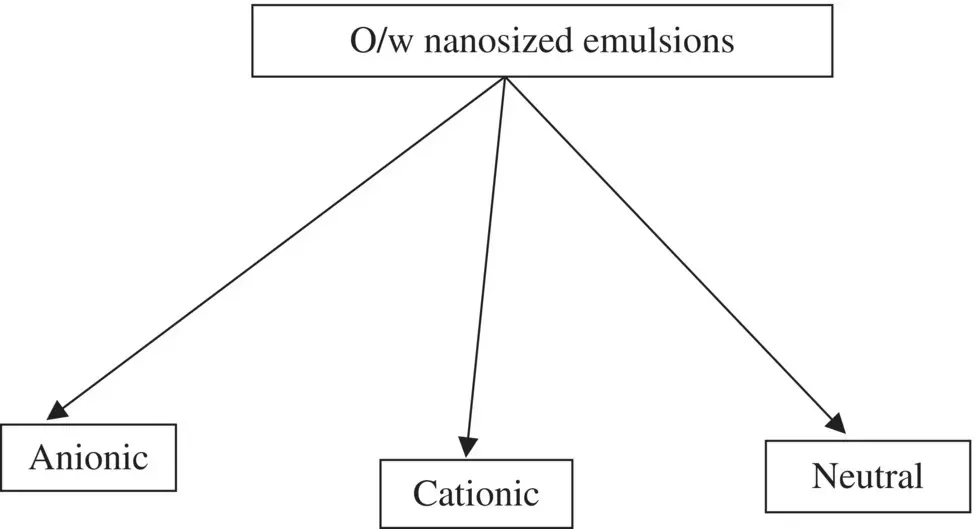
Figure 1.2. Classification of oil‐in‐water (o/w) nanosized emulsions based on emulsifier molecules.
According to Capek (2004), the stability of the electrostatically‐ and sterically‐stabilized o/w nanosized emulsions can be controlled by the charge of the electrical double layer and the thickness of the droplet surface layer formed by non‐ionic emulsifier, respectively. In spite of the similarities between electrostatically‐ and sterically‐stabilized emulsions, there are large differences in the partitioning of molecules of ionic and non‐ionic emulsifiers between the oil and water phases and the thickness of the interfacial layers at the droplet surface (Capek 2004). The thin interfacial layer (the electrical double layer) at the surface of electrostatically stabilized droplets does not create any steric barrier for mass transfer. This may not necessarily be true for the thick interfacial layer formed by a non‐ionic emulsifier. The sterically‐stabilized oil droplets, however, can favor the transfer of materials within the intermediate agglomerates. Hence, the stability of electrosterically‐stabilized emulsion ( δ k) is controlled by the ratio of the thickness of the non‐ionic emulsifier adsorption layer ( δ ) to the thickness of the electrical double layer ( k −1) around the oil droplets (Capek 2004).
(1.5) 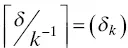
This section says that although the o/w nanosized emulsions belong to MS category in terms of stability aspects, many competing forces actually determine the stability of emulsions. The group of API molecules suitable to be incorporated into the o/w nanosized emulsions is carefully revived by interpreting the physicochemical properties (molecular size and structure, melting point, log P value, etc.) of individual APIs along with their solubility and permeability characteristics.
1 Anton, N., Benoit, J.‐P., and Saulnier, P. (2008), Design and production of nanoparticles formulated from nano‐emulsion templates—a review, J. Control. Release, 128(3), 185–199. doi:10.1016/j.jconrel.2008.02.007
2 Barkat, A.K., Naveed, A., Haji, M.S.K. et al. (2011), Basics of pharmaceutical emulsions: a review, Afr. J. Pharm. Pharmacol., 5 (25), 2715–2725. doi:10.5897/AJPP11.698
3 Bergström, C.A.S. and Larsson, P. (2018), Computational prediction of drug solubility in water‐based systems: qualitative and quantitative approaches used in the current drug discovery and development setting, Int. J. Pharm., 540 (1–2), 185–193. doi:10.1016/j.ijpharm.2018.01.044
4 Bergström, C.A.S., Wassvik, C.M., Johansson, K. et al. (2007), Poorly soluble marketed drugs display solvation limited solubility, J. Med. Chem., 50 (23), 5858–5862. doi:10.1021/jm0706416
5 Boverhof, D.R., Bramante, C.M., Butala, J.H. et al. (2015), Comparative assessment of nanomaterial definitions and safety evaluation considerations, Regul. Toxicol. Pharmacol., 73 (1), 137–150. doi:10.1016/j.yrtph.2015.06.001
6 Buscall, R., Davis, S.S., and Potts, D.C. (1979), The effect of long‐chain alkanes on the stability of oil‐in‐water emulsions. The significance of Ostwald ripening, Colloid Polym. Sci., 257, 636–644. doi:10.1007/BF01548833
7 Butler, J.M. and Dressman, J.B. (2010), The developability classification system: application of biopharmaceutics concepts to formulation development, J. Pharm. Sci., 99 (12), 4940–4954. doi:10.1002/jps.22217
8 Capek, I. (2004), Degradation of kinetically‐stable o/w emulsions, Adv. Colloid Interf. Sci., 107, 125–155. doi:10.1016/S0001‐8686(03)00115‐5
9 Chen, H., Chang, X., Weng, T. et al. (2004), A study of microemulsion systems for transdermal delivery of triptolide, J. Control. Release, 98 (3), 427–436. doi:10.1016/j.jconrel.2004.06.001
10 Chen, M.‐L., John, M., Lee, S.L., and Tyner, K.M. (2017), Development considerations for nanocrystal drug products, AAPS J., 19 (3), 642–651. doi:10.1208/s12248‐017‐0064‐x
11 Davis, S.S., Round, H.P., and Purewal, T.S. (1981), Ostwald ripening and the stability of emulsion systems: an explanation for the effect of an added third component, J. Colloid Interf. Sci., 80 (2), 508–511. doi:10.1016/0021‐9797(81)90210‐1
12 Davis, S.S. and Smith, A. (1973), Emulsion Theory and Practice, Academic Press, London.
13 Donaldson, K. and Poland, C.A. (2013), Nanotoxicity: challenging the myth of nano‐specific toxicity, Curr. Opin. Biotechnol., 24 (4), 724–734. doi:10.1016/j.copbio.2013.05.003
14 Dunning, W.J. (1973), Particle growth in suspensions, No. 38 in SCI monograph, Academic Press, London.
15 Goldberg, A.H. and Higuchi, W.I. (1969), Mechanisms of interphase transport. II. Theoretical considerations and experimental evaluation of interfacially controlled transport in solubilized systems, J. Pharm. Sci., 58 (11), 1341–1352. doi:10.1002/jps.2600581109
16 Higuchi, W.I. and Misra, J. (1962), Physical degradation of emulsions via the molecular diffusion route and the possible prevention thereof, J. Pharm. Sci., 51, 459–466. doi:10.1002/jps.2600510514
17 Jain, N. and Yalkowsky, S.H. (2001), Estimation of the aqueous solubility I: application to organic nonelectrolytes, J. Pharm. Sci., 90(2), 234–252. doi:10.1002/1520‐6017(200102)90:2%3C234::aid‐jps14%3E3.0.co;2‐v
18 Junghanns, J.U.A.H. and Müller, R.H. (2008), Nanocrystal technology, drug delivery and clinical applications, Int. J. Nanomed., 3 (3), 295–309. doi:10.2147/ijn.s595
19 Kabalnov, A.S., Pertsov, A.V., Aprosin, Y.D. et al. (1985), Influence of nature and composition of disperse phase on stability of direct emulsions against transcondensation, Kolloidn. Zh. (Engl. Transl.), 46, 965–967.
20 Kabalnov, A.S., Pertsov, A.V., and Shchukin, E.D. (1987), Ostwald ripening in two‐component disperse phase systems: application to emulsion stability, Colloids Surf., 24, 19–32. doi:10.1016/0166‐6622(87)80258‐5
21 Kahlweit, M. (1975), Ostwald ripening of precipitates, Adv. Colloid Interf. Sci., 5, 1–35. doi:10.1016/0001‐8686(75)85001‐9
22 Keck, C.M. and Müller, R.H. (2006), Drug nanocrystals of poorly soluble drugs produced by high pressure homogenisation, Eur. J. Pharm. Biopharm., 62 (1), 3–16. doi:10.1016/j.ejpb.2005.05.009
23 Lallemand, F., Felt‐Baeyens, O., Basseghir, K. et al. (2003), Cyclosporine A delivery to the eye: a pharmaceutical challenge, Eur. J. Pharm. Biopharm., 56, 307–318. doi:10.1016/S0939‐6411(03)00138‐3
24 Lifshitz, I.M. and Slezov, V.V. (1959), Kinetics of diffusive decomposition of supersaturated solid solutions, Soviet Phys. J. Exp. Theor. Phys., 35 (8), 331.
25 Lifshitz, I.M. and Slezov, V.V. (1961), The kinetics of precipitation from supersaturated solid solutions, J. Phys. Chem. Solids, 19 (1–2), 35–50. doi:10.1016/0022‐3697(61)90054‐3
26 Lipinski, C.A. (2005), Solubility in water and DMSO: issues and potential solutions, in: Borchardt, R., Kerns, E., Lipinski, C., Thakker, D., and Wang, B., Eds., Pharmaceutical Profiling in Drug Discovery for Lead Selection, AAPS Press, Arlington, pp. 93–125.
27 Margreiter, R. (2002), Efficacy and safety of tacrolimus compared with ciclosporin microemulsion in renal transplantation: a randomised multicentre study, The Lancet, 359 (9308), 741–746. doi:10.1016/s0140‐6736(02)07875‐3
28 Medlicott, N.J., Waldron, N.A., and Foster, T.P. (2004), Sustained release veterinary parenteral products, Adv. Drug Deliv. Rev., 56 (10), 1345–1365. doi:10.1016/j.addr.2004.02.005
Читать дальше