The emulsion generated by the low‐energy or spontaneous emulsification process is termed as microemulsion. On the other hand, the emulsion produced by means of the high‐energy homogenizers is called as nanoemulsion or nanosized emulsion. Again there are few basic differences between microemulsion and nanosized emulsion. Firstly, the microemulsion possesses the dispersed phase droplet diameter value well below the range of 100 nm, typically even below 10 nm as well. But the dispersed phase droplet diameter of the nanosized emulsion lies above 100 nm that too in between 300 to 500 nm level. Secondly, the notable difference between these two emulsions is being their physical appearances. While the microemulsion creates solution like transparent appearance, the nanosized emulsion looks like slightly bluish‐coloured white milk. Thirdly, a moderately higher amount of single or multiple emulgent molecules is used for producing the microemulsion when compared to the emulsifier molecules' amount which is being utilized to prepare the nanosized emulsion. Table 1.2depicts the basic differences between microemulsion and o/w nanosized emulsion. In spite of these basic differences between microemulsion and o/w nanosized emulsion, these two terms (micro‐ and nano‐emulsions) are, however, used interchangeably in many medical and pharmaceutical literatures.
TABLE 1.2. Typical Differences Between Microemulsion and Oil‐in‐Water Nanosized Emulsion
| Microemulsion |
Oil‐in‐Water Nanosized Emulsion |
| Emulsifier concentration is high, i.e., 30–40% |
Emulsifier concentration is comparatively less, preferably 2–5% and can be increased up to 10% |
| It is a thermodynamically stable isotropic mixture |
It is thermodynamically unstable but kinetically stable for some time with the help of emulsifier molecule's coverage onto the dispersed oil droplet surface |
| Particle size ranges below 10 nm or below 100 nm |
Particle size ranges from 200 to 700 nm |
| It is toxic for parenteral application but oral dosage form is possible. Any water‐soluble API can be used |
All routes of application are possible |
1.1.2.1. Nanosized Emulsions: Prime Candidates for Nanoparticle Engineering
As discussed above, emulsions exist as two types often reported as being the same, thermodynamically stable (microemulsion) and thermodynamically unstable or metastable (MS) varieties (nanosized emulsion), the prevailing form is a function of surface tension that is related to dispersion entropy. The factors that are critical in long‐term product stability are presented in flowchart form ( Flowchart 1.2). The MS or nanosized emulsions are considerably more susceptible to the destabilization phenomena than thermodynamically stable variety. Destabilization does occur due to aggregation or coalescence of dispersed oil droplets of o/w nanosized emulsion and Ostwald ripening phenomena. Whereas the aggregation or coalescence destabilization phenomena are related to the mechanics of the droplet surface, the Ostwald ripening describes the change of an inhomogeneous structure or size in the dispersed oil droplets of emulsion over time. The presence of inhomogeneous sizes in the dispersed oil droplets allow the smaller particles to dissolve and deposit on the larger particles in order to reach a more thermodynamically stable state wherein the surface to area ratio is minimized, i.e., the formation of larger‐sized dispersed oil droplets at the expense or unison of smaller‐sized oil droplets. This is accentuated by the smaller particle dimensions because of the increased internal Laplace pressure that is related to droplet surface curvature. The Ostwald ripening is otherwise termed as particle coarsening. This causes interfaces with low curvature to grow at the expense of interfaces with high curvature and the average size‐scale of the domains to increase in size with time. The driving force for this process is the minimization of the total interfacial energy of the system. Coarsening typically occurs under conditions where the volume fractions of the phases are nearly at their equilibrium values. The coarsening process has a profound effect on the properties of materials. For example, the Ostwald ripening or particle coarsening impacts directly on continuous phase solubility of the dispersed phase components and encapsulated API.
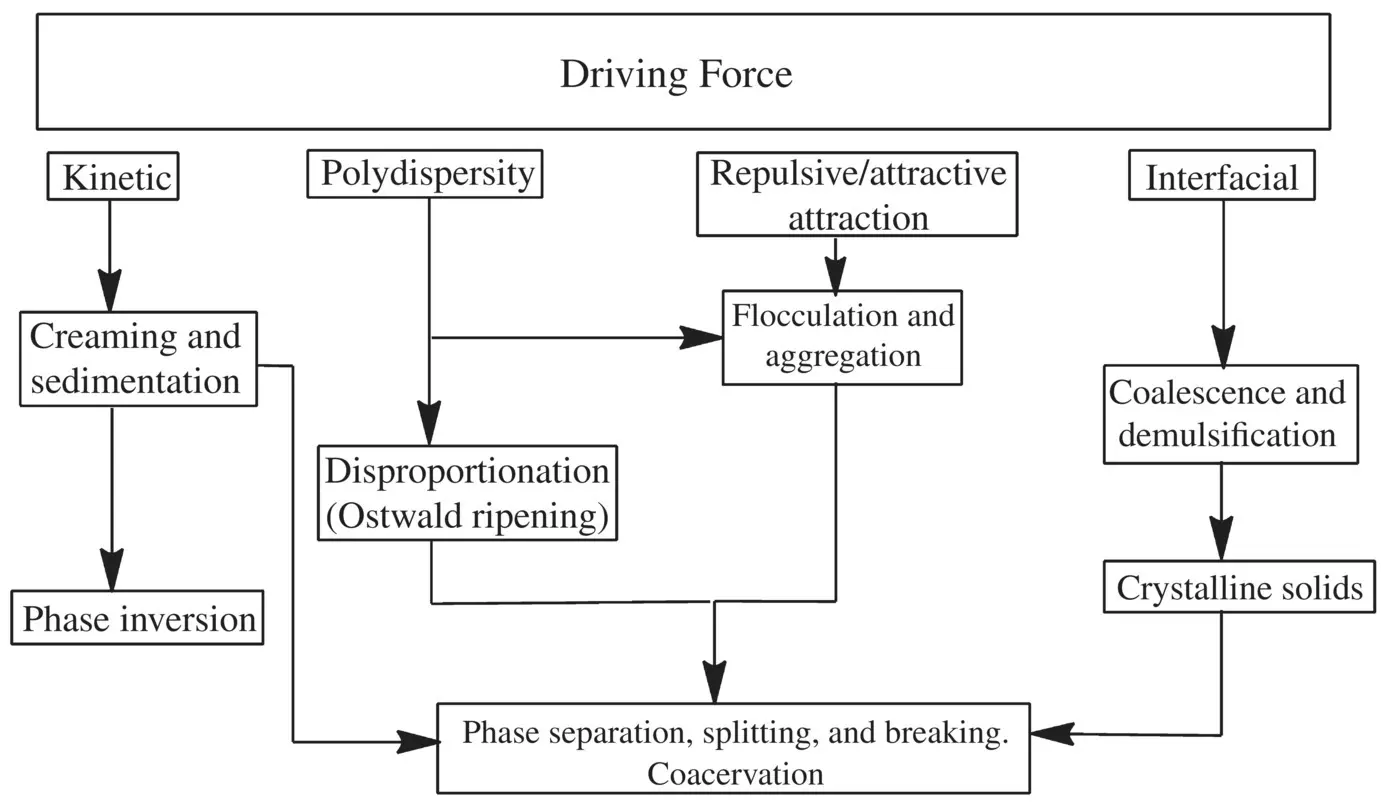
Flowchart 1.2. Critical factors to determine long‐term product stability.
The manufacturers of new colloidal products (nanosized emulsions) have to be aware of the best manufacturing techniques, limitations of producing new products and efficacy of the final administered product (Margreiter 2002). Because emulsions that are easy to produce on a laboratory‐scale can be much more difficult to mass produce. The lab‐designed product does not always come with an acceptable range of built‐in invulnerability to variable storage conditions and viable shelf life of the final marketed product. Worryingly, MS nanosized emulsion droplets are notable for the size‐related improved stability and with smaller sizes the formulated products show better medium‐term stability (Medlicott et al. 2004). They are also worth considering for regular use since they have rapid API release and viable API partition coefficients (Chen et al. 2004), say from interior to interface and thus to bulk, which is primarily because of the exceptionally large surface area of nanospheres. Furthermore, adaptation is possible as the surface fluidity of interface emulsifiers can be selectively modified. In terms of API delivery, nanosized emulsions have been reported to have a particular favourable predisposition for vascular wall and capillaries (Mizushima 1996).
The o/w nanosized emulsions are nanometric‐sized emulsions, typically exhibiting diameters of up to 500 nm or often quoted as being 400–800 nm. However, particles in this range tend to be thermodynamically unstable non‐self forming requiring mechanical energy input and are opaque. Nanosized emulsions are also frequently known as miniemulsions, fine‐dispersed emulsions, submicron emulsions and so forth, but are all characterized by a great stability in suspension due to their very small size, essentially the consequence of significant steric stabilization between droplets, which goes to explain why the Ostwald ripening is the only adapted droplet destabilization process (detailed below).
1.1.2.2. Ostwald Ripening‐Adapted Droplet Destabilization Process for the Greater Stability of Nanosized Emulsions
The main particularity of nanosized emulsions, making them prime candidates for nanoparticle engineering, is their great stability of droplet suspension. A kinetic stability that lasts for months, stability against dilution or even against temperature changes, totally unlike the (thermodynamically stable) microemulsions. Emulsions are thermodynamically unstable systems, due to the free energy of emulsion formation (Δ G f) greater than zero. The large positive interfacial energy term ( λ Δ A ) outweighs the entropy of droplet formation ( T Δ S f), also positive. The terms λ and Δ A , respectively represent the surface tension and the surface area gained with emulsification. Emulsion instability is therefore induced by the positive sign of Δ G f[ Eq. (1.2)].
(1.2) 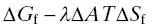
Читать дальше