In other words, Yalkowsky and coworkers established the General Solubility Equation (GSE), in which the solubility of a compound is expressed as a function of the melting point ( T m) and its lipophilicity (in the form of the octanol‐water partition coefficient, log K o/wor simply the log P value) (Jain and Yalkowsky 2001). The GSE states the following relationship:
(1.1) 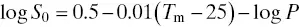
where S 0is the intrinsic solubility, i.e., the solubility of the non‐ionised (neutral species).
Specifically, “grease balls” represent highly lipophilic compounds (log K o/w> 3), which are poorly hydrated and their solubility is solvation limited, whereas “brick dust” compounds display lower lipophilicity and higher melting points ( T m> 200°C) and their solubility is limited by the strong intermolecular bonds within the crystal (Bergström et al. 2007), therefore, they said to have solid‐state‐limited solubility. Collectively, the APIs with solvation‐limited solubility are lipophilic, relatively large molecules and lack conjugated systems. Most of these grease ball molecules have been developed as oral dosage forms, however, the developed dosage forms typically include several different excipients that may improve dissolution (disintegration and dispersion) and solubilization. This indicates that an extensive API development process would be required to bring these grease ball molecules to the market. In contrast, the APIs with solid‐state‐limited solubility (brick dust molecules) are often flat, typically with an extended ring structure and display high aromaticity. These molecular features are important for forming a more stable crystal lattice. Hence, brick dust molecules have been found to benefit from formulation approaches such as particle size reduction and amorphization, whereas grease balls can be formulated as lipid or oil‐based formulations (Tuomela et al. 2016). Table 1.1shows the various properties of brick dust and grease ball APIs.
It should be noted, however, that while compounds described as solvation‐limited (grease ball) often display intrinsic solubility values in the lower nanomolar scale, none of the compounds included in the solid‐state‐limited (brick dust) category had intrinsic solubility values even in this lower nanomolar scale (Wassvik et al. 2008). Interestingly, griseofulvin which has a solubility of approximately 15 μM was the least soluble compound found in the dataset published by Wassvik et al. (2008). Two fundamental explanations may be given for why this is the case. First of all, after using the GSE relationship [ Eq. (1.1)] along with the knowledge that compounds with a log P value of 2 or less will tend to have solid‐state‐dependent solubility, the estimated or expected intrinsic solubility value of compounds having extraordinarily high melting points (of about 325°C) would be about 30 μM. Secondly, most of the solely solubility‐limited compounds because of their solid‐state are terminated relatively early in the API development process. So, in the quest of affordable APIs for therapeutic activity, the grease ball molecule looks competitively better than the brick dust molecule during the API or dosage form development process.
TABLE 1.1. Typical Differences Between Grease Ball and Brick Dust Molecules
Extracted from Bergström and Larsson (2018).
| Grease Ball API |
Brick Dust API |
| Highly lipophilic compound with high log P (>3 or 4) and a low melting point (<200°C) |
Compound with high log P (<2) and high melting point (>200°C) |
| Poorly soluble compounds restricted in solubility by poor hydration are described as grease ball molecule |
Compound with strong intermolecular bonds and/or complex interaction patterns with large number of interaction points between the molecules in the crystal lattice which often shows a limited capacity to dissociate from the solid form. This type of compounds are called as brick dust (stone‐like) |
| These compound cannot form bonds with water molecules, thus their solubility is limited by the solvation process |
The solubility of compounds in water is restricted due to strong intermolecular bonds within the crystal structure |
| Usual formulation approaches do not work, solubility enhancement through the use of a polar promoiety may prove useful |
If the molecule has brick dust nature, a polar promoiety may work as this strategy which might disrupt the intermolecular interactions that led to the high crystallinity |
| Grease ball APIs are the candidates for entrapment into various lipid‐and oil‐based nanoformulations |
Brick dust fraction that dissolves neither in oil nor in water cannot be administered as self‐emulsifying API delivery system |
Preparing the pulverized API suspended in aqueous or non‐aqueous medium (is termed as API nanosuspensions) is being suggested as a universal delivery approach for those group of orally administrable APIs that fall into class II (low solubility and high permeability) and class IV (low solubility and low permeability) of the Biopharmaceutics Classification system (BCS) (Keck and Müller 2006; Shegokar and Müller 2010). Another elegant way proposed by Butler and Dressman (2010) to classify the API molecules is the Developability Classification System (DCS) as this way of categorizing the API molecules is in a more biorelevant manner. According to the DCS, the API molecules can further be sub‐categorized into two types to distinguish between dissolution rate‐limited (DCS Class IIa) and solubility‐limited (DCS Class IIb) API molecules (as shown in Flowchart 1.1). More importantly, the intrinsic solubility and the related intraluminal API concentration for API molecules belonging to Class IIb and IV are too low to achieve sufficient flux over the epithelial membrane. Therefore, the API molecules belonging to DCS Class IIb and IV often utilize the complexation or other formulation approaches based on solid‐state modifications and even these approaches might be preferable compared with nanocrystals or nanosuspensions (Chen et al. 2017; Möschwitzer 2013; Shah et al. 2016).
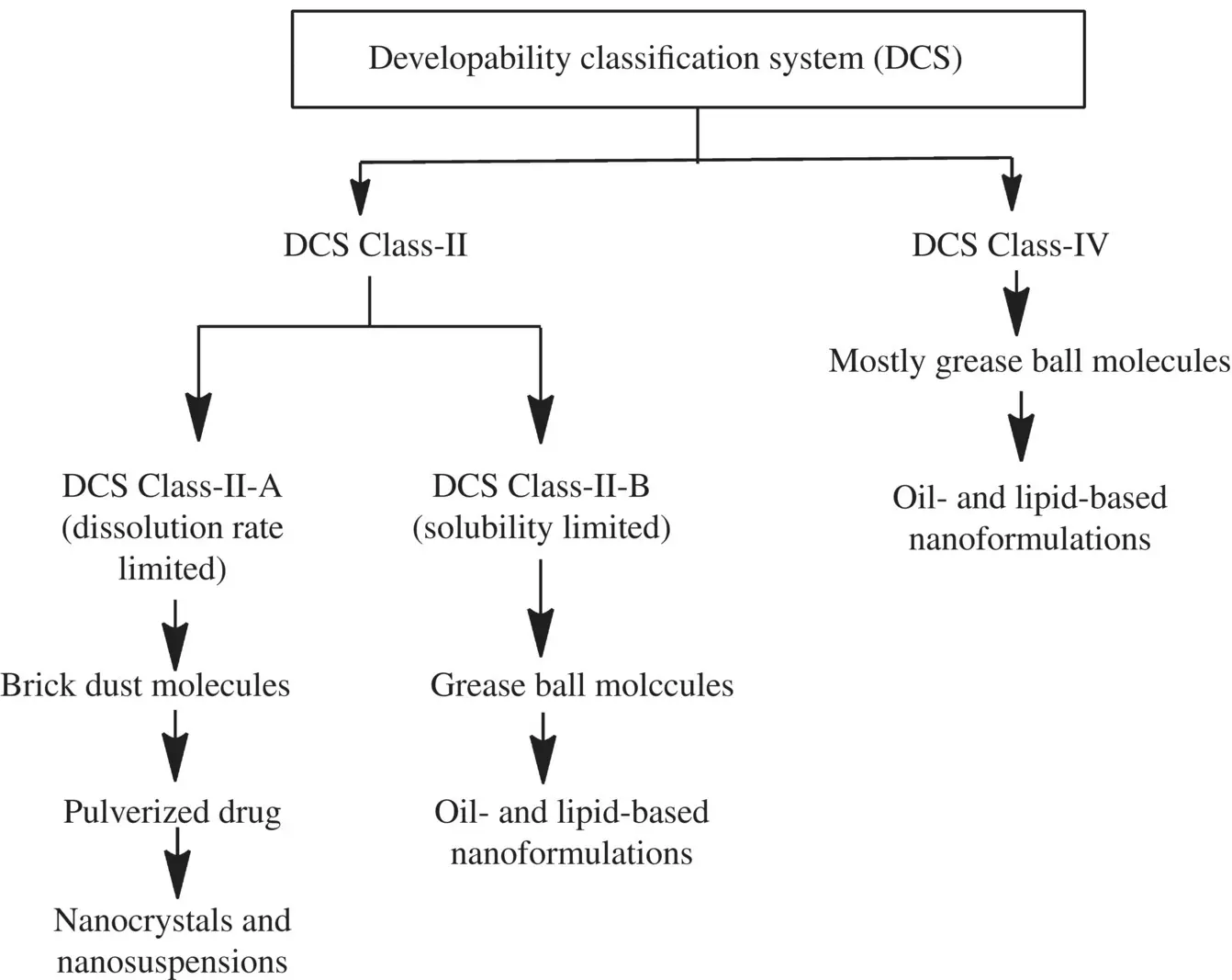
Flowchart 1.1. API sub‐categorization.
After understanding the clear‐cut difference between the grease ball and brick dust API molecules, the oil‐based heterogeneous, dispersion (liquid‐retentive) system, like emulsion, is the main centre of focal point to solve the solubility (and thus the intestinal permeability) problems of grease ball API molecules.
1.1.2. Nanosized Emulsions
An emulsion is a biphasic liquid preparation consisting of two immiscible liquids, one of which (the dispersed phase) is finely and uniformly dispersed as globules throughout the second phase (the continuous phase) (Barkat et al. 2011). If the amount of oil phase is significantly low when compared to the amount of water phase, then, the final emulsion is termed as oil‐in‐water (o/w) emulsion. Conversely, when the amount of water phase is significantly lower than the oil phase, the resulting emulsion system appears to be somewhat more viscous and is called as water‐in‐oil (w/o) emulsion. Both o/w and w/o type of emulsion systems are stabilized against the aggregation, coalescence and separation of dispersed oil or water phase by the addition of a third component called as emulsifying agent or emulgent or emulgator. In fact, the therapeutic emulsions are being stabilized by the addition of more than one emulgent molecules in order to prevent random collision of‐and then the coalescence of‐dispersed oil or water phase of the o/w or w/o emulsion. Mixing of appropriate amounts of oil, water and emulgent leads to the formation of an emulsion and this whole process is being named as emulsification. Apart from the amount of dispersed oil or water phase which will determine the type of final emulsion formed (whether o/w or w/o), the amount of single or multiple emulgent molecule added during the emulsification process will obviously control the type of emulsion produced. In addition, the size‐reduction equipments such as high‐energy or low‐energy homogenizer used to mix the oil and water phases along with single or multiple emulgent molecules will also direct the final emulsion produced in terms of mean size of the dispersed phase in the final emulsion. Interestingly, both high‐and low‐energy homogenizers will generate emulsions with nano‐range droplets particle sizes of the dispersed phase.
Читать дальше