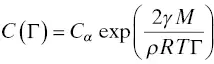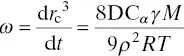Accordingly, the physical destabilization of emulsions is related to the spontaneous trend towards a minimal interfacial area between the two immiscible phases. Therefore, a minimization of interfacial area is attained by two mechanisms:
1 Flocculation followed mostly by coalescence, and
2 Ostwald ripening.
In nanosized emulsion systems, flocculation is naturally prevented by steric stabilization, essentially due to the sub‐micrometric droplet size. In short (Napper 1983; Tadros 1982; Tadros et al. 2004), when interfacial droplet layers overlap, steric repulsion occurs, from two main origins. The first one is the unfavorable mixing of the stabilizing chain of the adsorbed layer, depending on the interfacial density, interfacial layer thickness δ , and Flory–Huggins parameter χ 1,2(which reflects the interactions between the interfacial layer and solvent). The second one is the reduction of the configurational entropy, due to the bending stress of the chains, which occurs when inter‐droplet distance h becomes lower than δ .
Generally, the sum of the energies of interaction U Tadopts a typical shape of systems wherein molecules repel and particles attract each other, showing a weak minimum, around h = 2 δ , and a very rapid increase below this value (see Fig. 1.1for illustration). The depth of the minimum | U 0| will induce predispositions for coagulate in the colloidal suspension, that is to say, it is intimately linked to the stability of the suspension. | U 0| is shown to be dependent on the particle radius, the Hamaker constant A , and the adsorbed layer thickness δ , with the result that the higher the δ / r ratio, the lower the value of | U 0|. Now, in the case of nanoemulsion droplets, δ / r becomes very high in comparison with macroemulsions, which in the end totally inhibits its ability to coagulate. On the other hand, it is worth noting that the small droplet sizes also induce stabilization against sedimentation or creaming, in so far as the droplets are solely under the influence of the Brownian motion.
Taking all this into account, the destabilization of nanosized emulsions is due only to a mass transfer phenomenon between the droplets through the bulk phase, well described in the literature (Taylor 1998) as Ostwald ripening in emulsions. At the origin of this destabilization process, the differences, however slight, of the droplet radius induce differences in chemical potential of the material within the drops. The reduction of free energy in the emulsion will result in the decrease of the interfacial area, and therefore in the growth of the bigger emulsion droplets at the expense of the smaller ones. The dispersed phase migrates through the bulk from the smaller droplets to the bigger ones, owing to the higher solubility in the bulk of the smaller droplets. Ostwald ripening is initiated and will increase throughout the process. As an illustration and under the assumption that only one component composes the dispersed phase, the solubility, C (Γ), of the dispersed material throughout the dispersion medium is expressed as a function of the droplet radius r , from the Kelvin equation (Skinner and Sambles 1972) [ Eq. (1.3)],
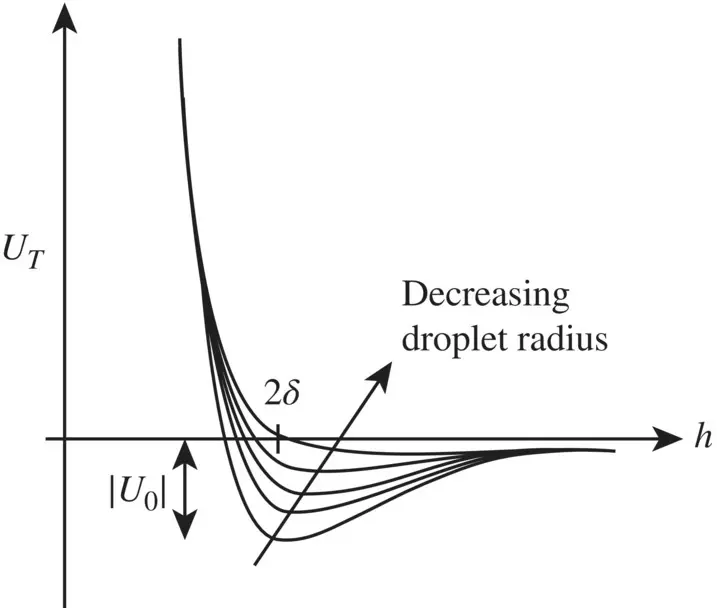
Figure 1.1. Influence of emulsion droplet radius on steric stabilization.
[Adapted from Anton et al. (2008).]
(1.3) 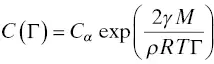
where C ∞is the bulk solubility of the dispersed phase, M its molar mass, and ρ its density.
In most studies, the follow‐up of Ostwald ripening as the temporal evolution of the droplet diameter still remains well fitted, even under the approximations involved in Eq. (1.3). In addition, the literature provides a number of theories dealing with calculations of the rate of ripening, such as the most famous (and complete) given by Lifshitz and Slezov (1959, 1961) and Wagner (1961), the so called LSW theory. Besides the consideration of Eq. (1.3), the diffusion of dispersed materials through the continuous medium is assumed to be diffusion‐controlled, i.e., crossing the interface with ease. Details on LSW theories are fully developed and discussed in the literature (Dunning 1973; Kahlweit 1975; Taylor 1998) leading to the commonly used expression of the ageing rate, ω , in Eq. (1.4),
(1.4) 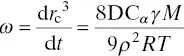
where r cis the critical radius of the system at any given time, at the frontier between the growth and decrease of the droplets. Consequently, Ostwald ripening is reflected by a linear relationship between the cube radius and time.
In processes involved in nanoparticle engineering, i.e., for multi‐component emulsion droplets, by adding monomer, polymer, or simply surfactant or co‐surfactant, the above approximation is surpassed. The rate of ripening can be reduced by several orders of magnitude when the additive has a substantially lower solubility in the bulk phase than the main component of the droplet. This phenomenon has been widely studied (Buscall et al. 1979; Davis and Smith 1973; Davis et al. 1981; Higuchi and Misra 1962; Kabalnov et al. 1985, 1987; Smith and Davis 1973; Taylor and Ottewill 1994), since it appears to be an efficient method to reduce the Ostwald ripening rate, even when using small amounts of additives. In short, it is explained by the difference of solubility in the continuous phase between the dispersed phase noted (1) and the additive (2), less soluble in this example. The first step remains similar to the ripening without additives, since only the component (1) diffuses from the smaller to the larger droplets, due to the higher chemical potential of the materials within the smaller drops. Gradually, the chemical potential in the larger droplets increases due to the presence of the component (2), until the diffusion process of (1) is stopped. Equilibrium is reached between the two opposing effects and the limiting process becomes the diffusion of the less soluble additive (2), significantly reducing the ripening rate and the nanoemulsion destabilization.
A final remark, which may be of importance here, concerns the influence on the nanosized emulsion destabilization of layer density and structure in the interfacial zone. Indeed, up to now it has been considered that Ostwald ripening is a diffusion‐controlled process, but this assumption does not take into account the fact that surfactants, polymeric emulsifiers or stabilizers can create a thick steric barrier at the droplet interface (Goldberg and Higuchi 1969; Yotsuyanagi et al. 1973). As a consequence, the diffusion of the inner material of the droplets may be slowed down, reducing the ripening rate. The substantial difference in stability between nanoemulsions and nanocapsules (another colloidal API delivery system having polymeric outer shells covered on the dispersed oil droplets) for instance, appears essentially from such details.
Before proceeding into Chapter 2, a brief description concerning classification of nanosized emulsions is presented below.
1.1.2.3. Classification of Oil‐in‐Water Nanosized Emulsions
Purely based on the emulsifier combinations used to stabilize the dispersed oil droplets of the emulsions, the o/w nanosized emulsions can be classified into three types ( Fig. 1.2). First type includes emulsions prepared using the emulsifiers that are having the capacity to assemble at the o/w interface and able to produce a minus (negative) charge in the vicinity of dispersed oil droplets of the emulsions. The emulsions thus formed are termed as anionic or negatively‐charged emulsions. The emulsions made with the inclusion of emulsifiers that are assembled at the o/w interface and competent to confer a plus (positive) charge in the vicinity of dispersed oil droplets of the emulsions are called as cationic or positively‐charged emulsions. The literature suggests that neither triglycerides nor phospholipidic emulsifier's components of the conventional or anionic emulsions are able to significantly sustain the incorporated lipophilic API release in simulated or real physiological environments under sink conditions. Therefore, in an attempt to prolong and/or optimize the API release, cationic lipid or polysaccharide emulsifiers are added to the emulsions to elicit mucoadhesion with anionic ocular tissues by an electrostatic adhesion. Indeed, cationic emulsions prepared on the basis of stearylamine, oleylamine and chitosan can serve this purpose. It was initially believed and now has become clearer from many reports in the literature that an occurrence of electrostatic attraction between the cationic emulsified droplets and anionic cellular moieties of the ocular and topical skin surface tissues enhance the bioavailability of emulsions containing lipophilic APIs (Lallemand et al. 2003; Piemi et al. 1999; Tamilvanan et al. 2002; Vandamme 2002). There is another type of emulsions that are neutral in terms of the charge on the dispersed droplets. These are instead stabilized through steric effects exerted by the emulsifier molecule present in the emulsion formulation.
Читать дальше