One applied strategy for stabilization of alkylamine in the structure of MOFs is grafting alkylamine ligands on open metal sites (OMSs) through post-synthesis procedure. Different types of alkylamine ligands like tetraethylenepentamine, N,N’-dimethylethylenediamine, 1-methylethylenediamine, 1,1-dimethylethylenediamine, ethylenediamine, piperazine, 3 and 4-picolylamine, N,N’-dimethylethylenediamine were grafted through this strategy to prove higher Q stand selectivity compared to arylamine functionalized MOFs [5–8, 26–33].
Immobilization of alkylamine ligands on open metal sites of MOFs is an ideal strategy to improve the affinity of the frameworks to CO 2molecules through altering the Lewis basic open metal sites to the Lewis basic alkylamine functionalized nodes. Based on this approach, the favorable CO 2physisorption by open metal site∙(O)carbon dioxide interaction change into strong CO 2chemisorption by alkylamine (N)∙(C)carbon dioxide interaction which leads to high CO 2adsorption enthalpy and selectivity. Homodiamine N,N’-dimethylethylenediamine (mmen) ligand applied in the structure of CuBTTri and Mg(dobpdc) 2to develop post-synthetically modified materials with high affinity to carbon dioxide molecules 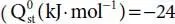 , −96, −47 and −71 for Cu-BTTri, mmen-Cu-BTTri, Mg(dobpdc) 2and mmen-Mg(dobpdc) 2respectively) [6, 34].
, −96, −47 and −71 for Cu-BTTri, mmen-Cu-BTTri, Mg(dobpdc) 2and mmen-Mg(dobpdc) 2respectively) [6, 34].
Originally, Mg-MOF-74 could adsorb exceptional amount of carbon dioxide (20.6 wt%) under relevant post-combustion flue gas conditions while it display only about 16% recovery of its initial amount of CO 2in breakthrough experiment with 70% humidity [35]. To remove this barrier and improvement of CO 2capacities at lower CO 2partial pressures and presence of humidity, Mg(dobpdc) 2post-synthetically modified with homodiamine ligands like ethylenediamine and dimethylethylenediamine [31]. Anyway, the working capacity of these alkylamine grafted FMOFs is not high at low temperatures. This is another issue that must be eliminated for efficient release of CO 2and increase in carbon dioxide working capacity.
Chang Seop Hong and coworkers synthesized a diamine-grafted FMOF (with the name of dmen-Mg 2(dobpdc) where dmen = N,N’ dimethylethylenediamine and H 4dobpdc = 4,4’-dihydroxy-(1,10-biphenyl)-3,3’dicarboxylic acid) and applied it for carbon dioxide capture and evaluation of working capacity at post-combustion conditions ( Figure 2.1) [8]. dmen is a heterodiamine with both primary and tertiary amines and incorporated into framework through post-synthesis modification. The results reveal that dmen-Mg 2(dobpdc) represent high CO 2/N 2selectivity (S = 554 at 25 °C and p(CO 2) = 0.15 bar) which is higher that mmen-Mg 2(dobpdc) (S = 200) and en-Mg 2(dobpdc) (S = 230). At 25 °C and 0.15 bar, activated dmen-Mg 2(dobpdc) adsorb 3.77 mmol·g −1of CO 2which is higher than en-Mg (dobpdc) (3.62 mmol·g −1) and mmen-Mg (dobpdc) (3.13 mmol·g −1). Efficiency of an adsorbent in post-combustion process is assessed by measuring the working capacity which is defined as difference between the adsorbed quantities at P ads= 0.15 bar CO 2/T ads= 40 °C and P des= 1 bar CO 2/T des. The higher working capacity at lower desorption temperatures, the lower energy consumption. dmen-Mg 2(dobpdc) could adsorb 18.8 wt% for pure CO 2, and 14.1 wt% for N 2/CO 2(85/15) which is close to that at the corresponding CO 2partial pressure in the isotherm at 40 °C, while no obvious adsorption was observed for pure N 2at 40 °C. Regeneration of the material is evaluated via vacuum-swing adsorption (VSA) and temperature-swing adsorption (TSA) methods. In TSA method, CO 2was adsorbed at 40 °C for 1 h and desorbed at 75 °C for 1 h under Ar. After 24 cycles, no capacity loss was observed, revealing that the dmen-Mg 2(dobpdc) is thermally stable under these experimental conditions as well as maintenance in its adsorption capacity. In VSA method, at 25 °C, material was saturated with CO 2at 1.2 bar and then placed under high vacuum. The removal of adsorbed CO 2from the solid was performed repeatedly by applying a vacuum to the adsorbent. Based on observed results, such a large amount of adsorbed CO 2(4.5 mmol·g −1) at 1.2 bar can be completely desorbed only under vacuum, without heating. The working capacities of dmen-Mg 2(dobpdc) at 130 to 90 °C desorption temperature is in the range of 11.7–13.5 wt%. Notably, experimental results reveal that at T des= 75 °C working capacity is 11.6 wt% which is higher that top performing MOFs such as Mg-MOF-74 (3.7 wt%), Mg 2(dobpdc) (4.5 wt%), en-Mg 2(dobpdc) (2.9 wt%), mmen-Mg 2(dobpdc) (2.1 wt%), and tmen-Mg 2(dobpdc) (3.9 wt%) (tmen = N,N,N’,N’-tetramethylethylenediamine). However, the working capacity of dmen-Mg 2(dobpdc) sharply reduced to almost zero at 70 °C. To evaluate the reusability of dmen-Mg 2(dobpdc) in the presence of water vapor, the material exposed to water vapor (100% RH for 10 min). Since the solid sample can be fully saturated with CO 2within the exposure time (10 min), it exposed to water vapors for 10 min. Then, dmen-Mg 2(dobpdc) reactivated under a pure CO 2flow at 130 °C for 4 h, followed by CO 2adsorption at 40 °C. After 5 cycles, a capacity loss of 5% is observed which is markedly higher than working capacities of the other MOFs during the first cycle (lower than 4.5 wt%). Clausius–Clapeyron equation applied for estimation of heat of adsorption (−Q st) so that increases to 75 kJ·mol −1at a loading of 0.25 mmol.g −1, and remains almost invariant in the range of 71–75 kJ·mol −1below loadings of 2.6 mmol.g −1. Based on DFT calculations, the open metals site are mostly occupied by the primary amine end of dmen-Mg 2(dobpdc), although some tertiary amine ends are probably grafted onto the exposed metal site as well. Possible mechanism based on DFT calculations and in - situ FT-IR analysis possible CO 2adsorption mechanism is illustrated in Figure 2.1f.
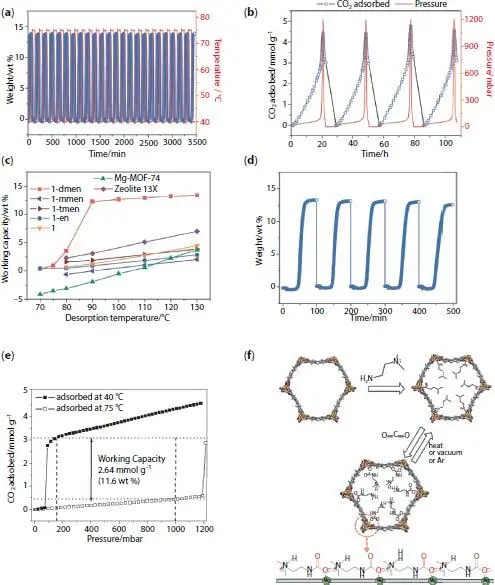
Figure 2.1 Application of dmen-Mg 2(dobpdc) in selective capture-release of carbon dioxide. (a) TSA process: Adsorption (40 °C)-desorption (75 °C) cycling of CO 2under Ar. (b) Vacuum-swing adsorption at 25 °C. (c) The working capacities of 1-dmen and the other porous solids obtained under the same conditions. (d) CO 2adsorption of 1-dmen in flue gas using the sequence (adsorption at 40 °C, desorption at 130 °C under pure CO 2-10 min exposure to 100% RH). (e) Estimated working capacity from q ads(P ads= 0.15 bar CO 2, T ads= 40 °C)-q des(P des= 1 bar CO 2, T des= 75). (f) Framework structure of 1 with open metal sites, grafting modes of dmen onto the open metal sites, and subsequent CO 2adsorption. The schematic diagram (bottom) indicates the arrangement of ammonium carbamates running along the c-axis [8].
Although extensive studies conducted on application of amine decorated MOFs is carbon dioxide capture and release, but there is an urgent need to clarify the effective conditions of arylamine or alkylamine groups in practical CO 2capture and release for real-life applications.
Christian Serre and coworkers applied amine decorated MIL-125(Ti) (MIL-125 formula is (Ti 8O 8(OH) 4(BDC) 6) where BDC is benzenedicarboxylate), denoted as NH 2-MIL-125(Ti) for separation of CO 2and H 2S [9]. They mentioned this material could improve separation of these acid gases from biogas or natural gas markedly. Based on in - situ FT-IR analysis, they mentioned that –NH 2function interact weakly with CO 2through lone pair of relatively negative N-atom of amine and relatively positively charged C-atom of CO 2. Also, hydrogen bonding is the main reason for improved H 2S separation in a way that H 2S acts as hydrogen bond donor and amine, through its N atom, acts as hydrogen bond acceptor.
Читать дальше

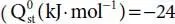 , −96, −47 and −71 for Cu-BTTri, mmen-Cu-BTTri, Mg(dobpdc) 2and mmen-Mg(dobpdc) 2respectively) [6, 34].
, −96, −47 and −71 for Cu-BTTri, mmen-Cu-BTTri, Mg(dobpdc) 2and mmen-Mg(dobpdc) 2respectively) [6, 34].











