Amine decorated FMOFs applied for Lewis basic catalyzed reactions like Henry and Knoevenagel reactions [38] and some of other reactions like transesterification of triglycerides [39]. Also, Amine decorated FMOFs with open metal sites applied in those kinds of tandem reactions needing both Lewis basic and Lewis acidic catalytic sites like cycloaddition reaction of CO 2with various epoxides [20].
In Henry or Knoevenagel reactions, benzaldehyde should be activated and then react with nitromethane or malonitrile, respectively. Since, benzaldehyde contains positively-charged C-atom which is Lewis acid site, Lewis basic sites can active benzaldehyde and catalyze Henry or Knoevenagel reactions. In one possible mechanism, it is reported that benzaldehyde can be activated through the interaction with an amine group and the formation of imine through a new (amine)N =C(benzaldehyde) covalent bond that can be followed by the rearrangement and addition of malonitrile ( Figure 2.6) [18]. In other mechanism it is mentioned that benzaldehyde activation in Henry reaction is done through a noncovalent Lewis base–acid interactions between Lewis basic catalytic site with the carbonyl C atom of benzaldehyde [40].
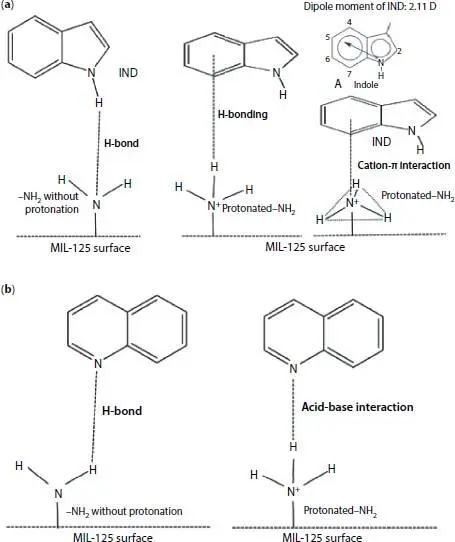
Figure 2.5 Application of NH 2-MIL-125 (Ti) and  -MIL-125 (Ti) in oil denitrogenation. (a) Adsorption mechanism for removal of indole. (b) Adsorption mechanism for removal of quinoline [15].
-MIL-125 (Ti) in oil denitrogenation. (a) Adsorption mechanism for removal of indole. (b) Adsorption mechanism for removal of quinoline [15].
Lewis basicity and hydrogen-bond donation/accepting are common and well-known chemical properties of amine functions which applied extensively in development of functional MOFs. Anyway, there are some of other chemical properties which are interesting for fabrication of FMOFs. For example, amine function is able to interact with donor acceptor interactions. Through this mechanism, amine decorated MOFs applied for improved Li-storage capacity [41] through host-guest interactions between Li and amine groups (N atoms) and accelerated I 2removal [42] through
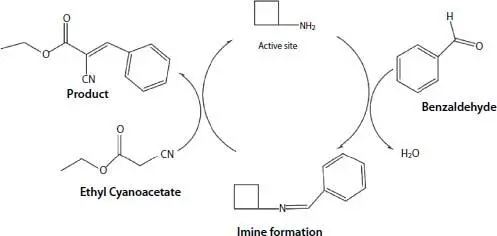
Figure 2.6 Possible catalytic mechanism by amine function in Knoevenagel reactions. In this mechanism, amine site could active benzaldehyde through formation of imine. Reaction is progressed after addition of ethyl cyanoacetate to the imine complex [18].
In synthetic organic chemistry it is known that aromatic rings with electron donor groups like amine could participate in electrophilic substitution reactions. Also, aromatic amine groups could be converted to diazonium or other products like reduction to hydrogen. These principal roles in chemistry of arylamines applied in construction of highly efficient removal and sensing of Cl 2[43], NO [44] and NO 2[45] gases with amine decorated MOFs.
Gregory W. Peterson and coworkers synthesized UiO-6-NH 2and applied in for removal of chlorine gas [43]. The material could remove 1.24g·g −1Cl 2(g). Using different characterization methods they proposed that there are two predominately mechanisms engaged in removal process including the loss of one carboxylate group of ligand and reaction between Cl 2(g) and Zr 6O 6nodes as well as reaction between organic linker (2-aminoterephthlic acid) and chlorine gas through electrophilic substitution reaction in ortho and para positions. Also, produced HCl molecules are neutralized by amine functions ( Figure 2.7a).
In another work by the same group, UiO-66-NH 2applied for removal of 1.4g·g −1NO 2(g) [45]. Experimental analyses show that NO 2(g) is adsorbed through different types removal mechanisms ( Figure 2.7b). At low loading, NO 2(g) first adsorbs within the pores of the MOF and loading increases with decreasing the temperature indicating that physical adsorption has a major impact on removal. At higher loading, the organic ligand react with oxidant NO 2(g) molecules in multiple locations.
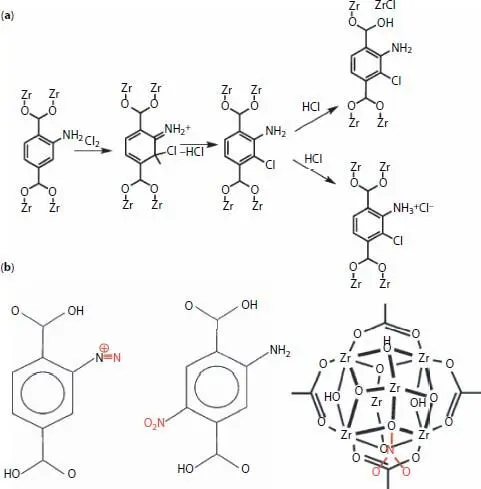
Figure 2.7 Application of UiO-66-NH 2in removal of harmful gases. Removal and degradation mechanism of chlorine (a) [43] and nitrogen dioxide (b) [45] gases on 2-aminoterphthalate linker of UiO-66-NH 2.
Sujit K. Ghosh and coworkers applied UiO-66-NH 2for aqueous phase detection of nitric acid gas ( Figure 2.8) [44]. After exposure to NO(g) and deamination process, UiO-66-NH 2is transformed to UiO-66. Considering this mechanism, fluorescent UiO-66-NH 2is converted to non-fluorescent UiO-66. PL measurements reveal that UiO-66-NH 2could detect NO(g) gas with detection limit equal to 0.575 µM and a quenching constant of 4.15 × 10 +5M −1. The UiO-66 framework do not show any change is PL emission peak which clarifies the role of the primary amine group in the NO(g) detection. Competitive experiments also show that there is no substantial change in presence of similar species while there is considerable quenching in presence of NO(g).
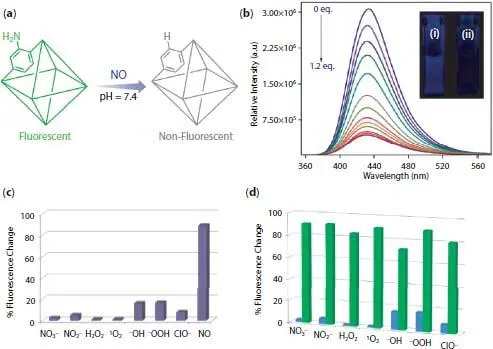
Figure 2.8 Application of UiO-66-NH 2in aqueous media detection of NO(g). (a) Proposed mechanism for detection of NO(g) molecules. (b) quenching in PL emission of UiO-66-NH 2after addition of NO(g). (c) Quenching efficiency in presence of other analytes. (d) Competitive experiment for detection of NO(g) in presence of other analytes [44].
Photoactive MOFs could be developed by immobilizing photoactive catalytic sites in MOF materials. Especially, practical adsorption of solar light could be easily attained by functionalization of the metal ions or the organic ligands. Amine function is recognized as a photosensitizer group in the structure of MOFs for the improvement of solar-light photocatalytic activity in MOFs [46–49]. 2-aminoterphthalic acid is well-known linker for construction of amine decorated MOFs like NH 2-UiO-66, NH 2-MIL-125 and other MOFs.
The amine function has a substantial role in the modification of optical band gap of MOFs constructed based on 2-aminoterphthalic acid ligand. In this case, HOMO of amine decorated MOFs based on 2-aminoterphthalic acid ligand composed of O, C and N 2p orbitals [50]. The insertion of N character in HOMO, or valance band, of MOFs induces the band-gap narrowing to shift the photo-absorption and lower band gap will shift the band gap to the visible light region [49]. So, the material shows an extended absorption band in the visible light region with enhanced visible-light absorption owing to introduction of photosensitizer amine function. Moreover, upon light irradiation a ligand-to-metal charge transfer with long-lived excited charge separation could be observed [47]. This charge transfer is effectual for oxidizing of reactive substrates adsorbed on the amine site by photogenerated holes on organic ligand and reducing other reactive substrates adsorbed on the inorganic building blocks by transferring of photogenerated electrons. So amine function could intensify the photocatalytic activity of MOFs through extending in absorption band and generation of long-lived excited electron-holes.
Jinhua Ye and coworkers synthesized MIL-88(Fe) and MIL-88(Fe)-NH 2and applied for photo-reduction of dichromate anion ( Figure 2.9) [47]. The mechanism of photo-reduction is based on generation of electron-hole pairs upon light irradiation. It is essential to tune the band gap of MOFs to optimize the photocatalytic activity of MOFs. In parent framework, MIL88(Fe), Fe 3-µ 3-oxo clusters are directly excited and reduction of Cr(VI) and oxidation of water take place at the Fe-based oxo clusters. But in case of MIL88(Fe)-NH 2, photo-sensitizer amine group enables organic ligand to adsorb the visible light photons, excited with generation of long-lived electronhole pairs and then transferring photoexcited electron to Fe 3-µ 3-oxo clusters. This secondary excitation mechanism is the reason of improved photocatalytic activity of MIL-88(Fe)-NH 2than its parent framework. Diffuse-reflectance UV/vis spectrum of these materials show that the introduction of amine group in the organic linker of iron(III)-based MOF can enhance its light absorption in the visible region. This observation indicates that the more amine group incorporated into the iron(III)-based MOF, the more electron–hole pairs can be generated via excitation of amine functionality under visible-light irradiation, which might lead to enhance photocatalytic activity. Transient photocurrent spectroscopy reveals that incorporation of amine group in the iron(III)-based MOF can enhance the photocurrent significantly indicating the fact that the separation efficiency of photoinduced electron-hole pairs and the lifetime of the photogenerated charge carriers are improved, and this can be explained by the excitation of amine-functionalized organic linker and then the excited electrons transfer to Fe 3 -μ 3 -oxo clusters. Combination of Electron spin resonance (ESR) for pure 2-aminoterohthalic acid ligand and MIL-88(Fe)-NH 2is an effective method to gain evidence about LMCT process. Pure ligand shows an ESR signal of g = 2.004 during the irradiation of visible light which is originated from amine group while such signal is not detected for the MIL-88(Fe)-NH 2. ESR signal for MIL-88(Fe)-NH 2at g = 1.994 is attributed to Fe(III) species in which decreases upon light irradiation and recovered when irradiation is stopped. The decrease of Fe(III) ESR signal intensity could be attributed to the trapping of electrons by Fe(III) site in Fe 3-μ 3-oxo clusters. The disappearance of the ESR signal at g value of 2.004 and the decrease of the ESR signal at g value of 1.996 suggest the electron transfer from excited amine group to Fe 3-μ 3-oxo clusters in NH 2-MIL-88B(Fe) irradiated with visible light.
Читать дальше


 -MIL-125 (Ti) in oil denitrogenation. (a) Adsorption mechanism for removal of indole. (b) Adsorption mechanism for removal of quinoline [15].
-MIL-125 (Ti) in oil denitrogenation. (a) Adsorption mechanism for removal of indole. (b) Adsorption mechanism for removal of quinoline [15].













