1 ...8 9 10 12 13 14 ...21 It is worth noting that a substantial amount of lignin decomposing aromatics is characterized by the structure of phenol substituted by inert methoxy and alkyl groups (structures such as guaiacol or creosol), making polycondensation or radical polymerization especially difficult. Thus, there are numerous studies on (i) introducing the reactive groups, which are promoting further polymerization reactions [74], (ii) utilization of the reactive ortho ‐ and para ‐sites of phenol for hydroxymethylation or obtaining novolac or resol‐type resin using formaldehyde chemistry [75] otherwise, (iii) connecting lignin‐derived compounds to make oligomers with additional functional groups [76]. Bimetallic Zn/Pd/C catalytic method for converting lignin via the selective hydrodeoxygenation ( T = 150 °C and 20 bar H 2, using methanol as a solvent) directly into two methoxyphenol products has been reported [77]. The compound characterized by the increased content of hydroxyl groups might be obtained using the above method, via the reaction of o ‐demethylation of 2‐methoxy‐4‐propylphenol and aqueous HBr. In the next step, propylcatechol is glycidylated to epoxy monomer ( Figure 1.16).
Other techniques described in the literature involve the ozone oxidation of Kraft lignin toward splitting its aromatic rings and generation of the muconic acid derivatives. The ozonized lignin ( Figure 1.17a) might then be dissolved in an alkali water solution and cross‐linked with the water‐soluble epoxy resin (glycerol polyglycidylether).
Another interesting synthesis described in the literature begins from the dissolution of alcoholysis lignin or lignin sulfuric acid in ethylene glycol and/or glycerin ( Figure 1.17b) [78]. Next, the hydroxyl group in the lignin molecule is reacted with succinic acid to convert the lignin into multiple carboxylic acid derivatives. In the last step, the resulting products react with epoxy compound (ethylene glycol diglycidyl ether [EGDGE]) in the presence of dimethylbenzyl amine as a catalyst to provide the cross‐linked epoxidized lignin resin. In the obtained curried epoxy material, lignin acts as a hard segment (increasing value of T gwith increasing lignin derivatives). Additionally, a slight decrease of T dwith increasing content of biocomponent in epoxy resin suggests that the thermal stability of obtained epoxy system is not affected by the presence of lignin derivatives.
Based on numerous studies, one can conclude that lignin is a very promising natural resource for replacement of bisphenol A in the synthesis of epoxy resins, as it has aromatic structure with hydroxyl, carboxylic acid, and phenolic functional groups, which can react with epichlorohydrin to form bio‐based epoxy resins. One of the biggest problems for commercial application of lignin's derivatives, because of its complex and multifunctional nature, is isolation and the synthesis of monomers.
Vanillin (4‐hydroxy‐3‐methoxybenzaldehyde) is an organic compound consisting of a benzene ring substituted with three functional groups: aldehyde –CHO, hydroxyl –OH, and methoxy –O–CH 3( Figure 1.18a).
It is a naturally occurring compound (in the form of its β‐D‐glucoside, Figure 1.18b) that can be directly obtained in the extraction process from the bean or seed pods of Vanilla planifolia , the tropical orchid presently cultivated in a number of tropical countries. Although this method has been known for centuries and it is still used, actually less than 1% of vanilla produced in the world is obtained in such a way [79]. Almost all vanillin is now synthesized much more cheaply through chemical processes. Synthetic vanillin is commercially available and is commonly used in both food and nonfood applications, in fragrances, as a flavoring in pharmaceutical preparations, as an intermediate in the chemical and pharmaceutical industries for the production of herbicides, antifoaming agents or drugs, and in household products, such as air fresheners and floor polishes. Synthetic or semisynthetic vanillin can be derived from two compounds: guaiacol and eugenol, both available from petrochemical sources or of natural origin.
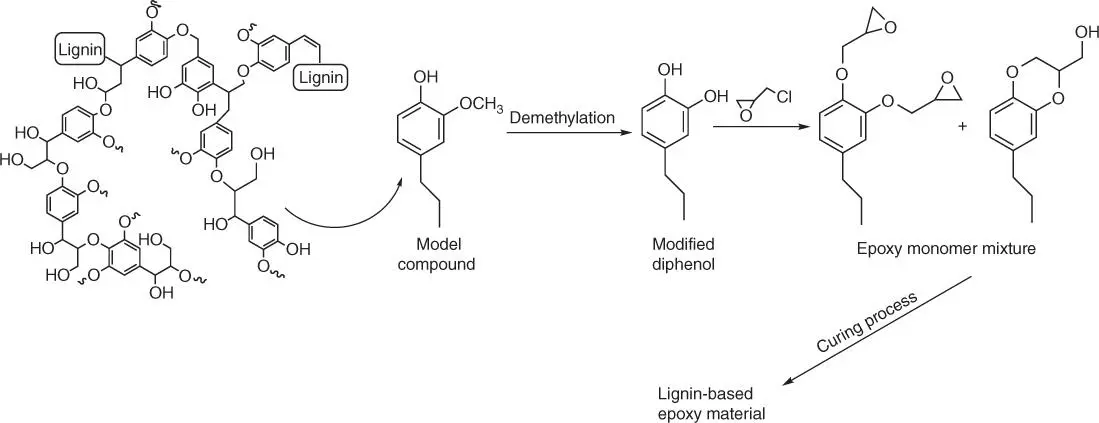
Figure 1.16 Route of the synthesis epoxy monomers from selectively hydrodeoxygenated lignin.
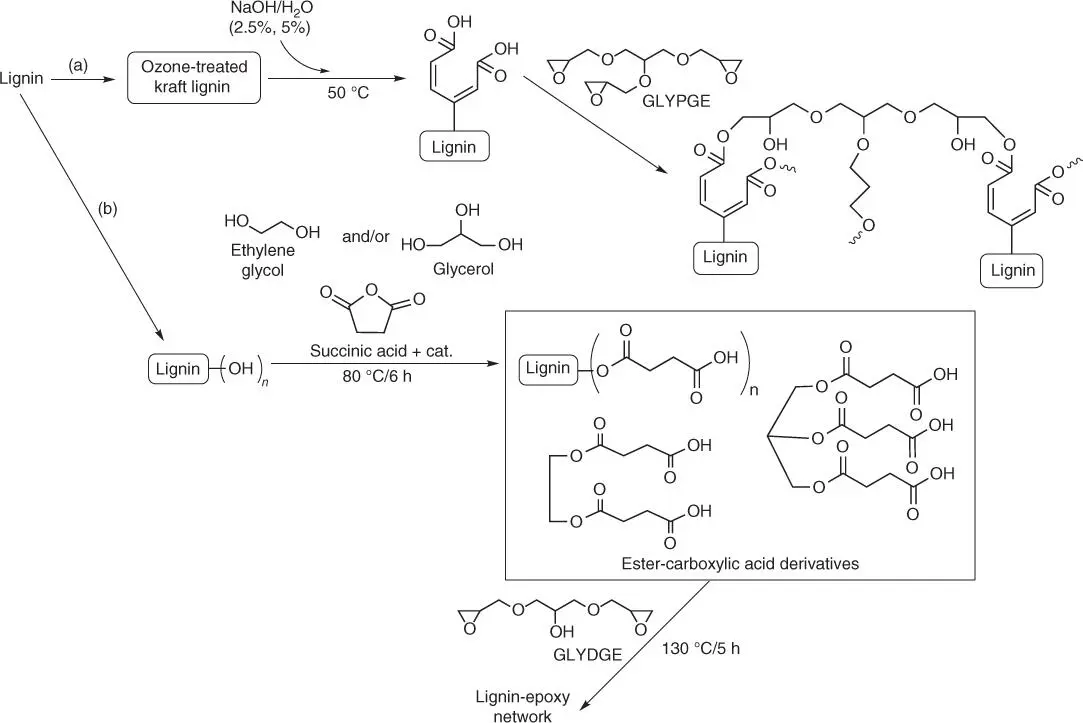
Figure 1.17 Lignin modification and cross‐linking: (a) ozone oxidation of Kraft lignin and (b) synthesis of multiple carboxylic acid derivatives.
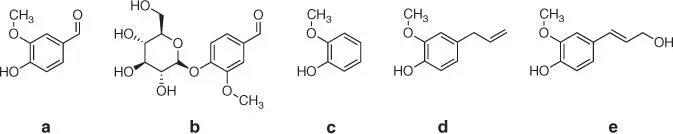
Figure 1.18 Chemical structures of ( a) vanillin and its naturally occurring precursors: ( b) vanillin glucoside, ( c) guaiacol, ( d) eugenol, and ( e) coniferyl alcohol.
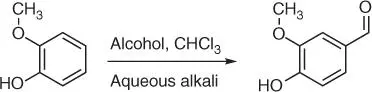
Figure 1.19 Synthesis of vanillin from guaiacol.
The first one, guaiacol (2‐methoxyphenol) ( Figure 1.18c), is a naturally occurring organic compound present in an aromatic oil from flowering plants Guaiacum . Guaiacol can also be gained from creosotes formed by distillation of various tars and pyrolysis of plant‐derived material, such as wood. Semisynthetic vanillin can be obtained from guaiacol through the Reimer–Tiemann reaction of phenols formylation ( Figure 1.19) [80].
The reaction is carried out using chloroform deprotonated by a strong base (hydroxide typically) to form the chloroform carbanion and finally the dichlorocarbene, which is the principal reactive specie in nucleophilic substitution also occurred in deprotonated phenol. Another method of vanillin synthesis from guaiacol is its reaction with glyoxylic acid ( Figure 1.20a), leading to the formation of 2‐hydroxy‐2‐(4‐hydroxy‐3‐methoxyphenyl)‐acetic acid ( Figure 1.20b) [81]. The obtained vanillylmandelic acid is converted via 2‐(4‐hydroxy‐3‐methoxyphenyl)‐2‐oxoacetic acid ( Figure 1.20c) to vanillin by the oxidative decarboxylation [82].
Eugenol (2‐methoxy‐4‐(prop‐2‐en‐1‐yl)phenol) ( Figure 1.18d) present in an essential oil extracted from the clove plant Syzygium aromaticum is the next important natural raw material for the vanillin synthesis ( Figure 1.21).
The synthesis consists of two steps: the basic isomerization of the double bond in eugenol leading to the formation of isoeugenol and oxidation of the rearranged double bond to vanillin [83, 84]. The process can be carried out with or without isolation of the intermediate product which is isoeugenol [85].
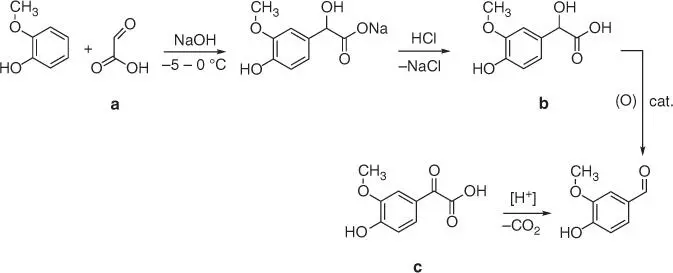
Figure 1.20 Synthesis of vanillin from guaiacol using glyoxylic acid.
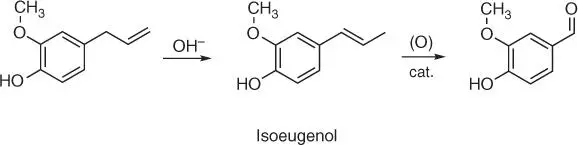
Figure 1.21 Synthesis of vanillin from eugenol.
Читать дальше


















