1 ...7 8 9 11 12 13 ...21 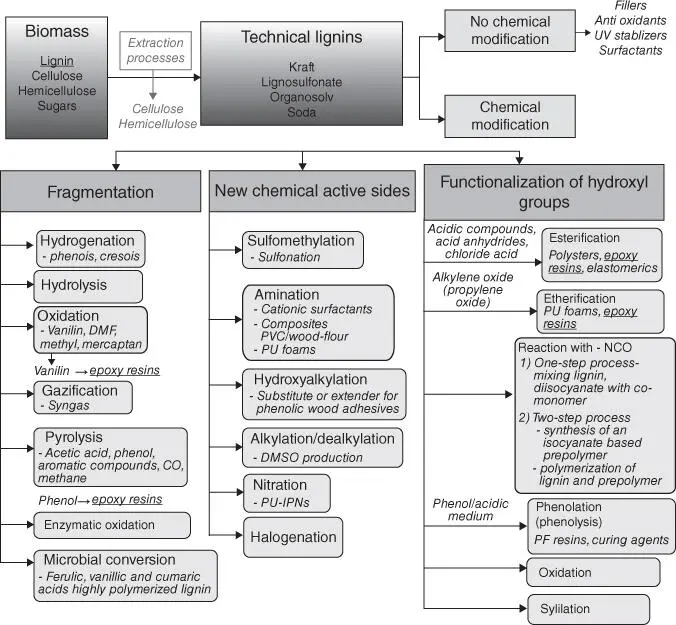
Figure 1.12 Summary of the main strategies for lignin conversion [49, 55].
Bio‐based epoxy resins are, for instance, synthesized from derivatives obtained on the course of lignin hydrogenolysis [71]. Lignin from pine wood is depolymerized by mild hydrogenolysis to give an oil product, containing aromatic polyols: dihydroconiferyl alcohol (DCA, 4‐(3‐hydroxypropyl)‐2‐methoxyphenol) and 4‐propyl guaiacol (PG, 4‐propyl‐2‐methoxyphenol), along with their dimers and oligomers. Then, the obtained oil product is dissolved in refluxing epichlorohydrin in the presence of solution of NaOH to give epoxy prepolymer (LHEP) ( Figure 1.13), which is then blended with bisphenol A diglycidyl ether (DGEBA) in mass ratios of LHEP : DGEBA up to 3 : 1. Epoxy composition might be cross‐linked with diethylenetriamine (DETA).
Table 1.3 Thermal analysis data for epoxy resin blends containing DGEBA and cured with DETA [68].
| Resin |
T g a )(°C) |
T 5% b )(°C) |
T s c )(°C) |
| DGEBA |
117 |
328 |
169 |
| LHEP/DGEBA 1 : 1 |
80 |
289 |
161 |
| LHEP/DGEBA 2 : 1 |
70 |
270 |
156 |
| LHEP/DGEBA 3 : 1 |
68 |
258 |
151 |
| LHEP |
53 |
236 |
144 |
a) T g– the glass transition temperature.
b) T 5%– the initial decomposition temperature.
c) T s– the statistic heat‐resistant index temperature.
Cured epoxy material LHEP/DGEBA is less thermally stable than the DGEBA resin ( Table 1.3) because of the presence of methoxy groups on the aromatic ring.
The initial decomposition temperature ( T 5%) and the statistic heat‐resistant index temperature ( T s) are the lowest for samples containing the highest proportion of LHEP. On the other hand, the presence of methoxy groups in the lignin hydrogenolysis products is likely to contribute to the superior mechanical properties of the cured LHEP/BADGE blends. Values of flexural modulus and strength of bio‐based materials are 52% and 28%, respectively, greater than DGEBA alone. Additionally, it is worth to mention here the research on the influence of the presence of lignin on the thermal performance and thermal decomposition kinetics of lignin‐based epoxy resins [72]. The presence of lignin‐based epoxy resin (depolymerized Kraft lignin, DKL‐epoxy resin, and depolymerized organosolv lignin, DOL‐epoxy resin, respectively) in epoxy composites, prepared by mixing the DGEBA and a desired amount (25, 50, and 75 wt%) of DKL‐epoxy resin and DOL‐epoxy resin at 80 °C, then cured with 4,4′‐diaminodiphenylmethane (DDM), results in a significant effect on the activation energy of the decomposition process, in particular, at the early and the final stage of decomposition ( Table 1.4).
The increase in the percentage value of lignin‐based epoxy resins in the composites reduces the initial activation energy of the system. Additionally, the obtained bio‐based materials exhibit higher limiting oxygen index (LOI) than that of the conventional BPA‐based epoxy resin, which might indicate that the lignin‐based epoxy composites are more effective fire retardants than the conventional BPA‐based epoxy resin.
An interesting example of a novel approach to finding new epoxy application for bio‐based derivatives from lignin is a conversion of lignin to epoxy compounds throughout the reaction of epichlorohydrin with partially depolymerized lignin (PDL) in the presence of benzyltriethylammonium chloride and dimethyl sulfoxide ( Figure 1.14) [67].
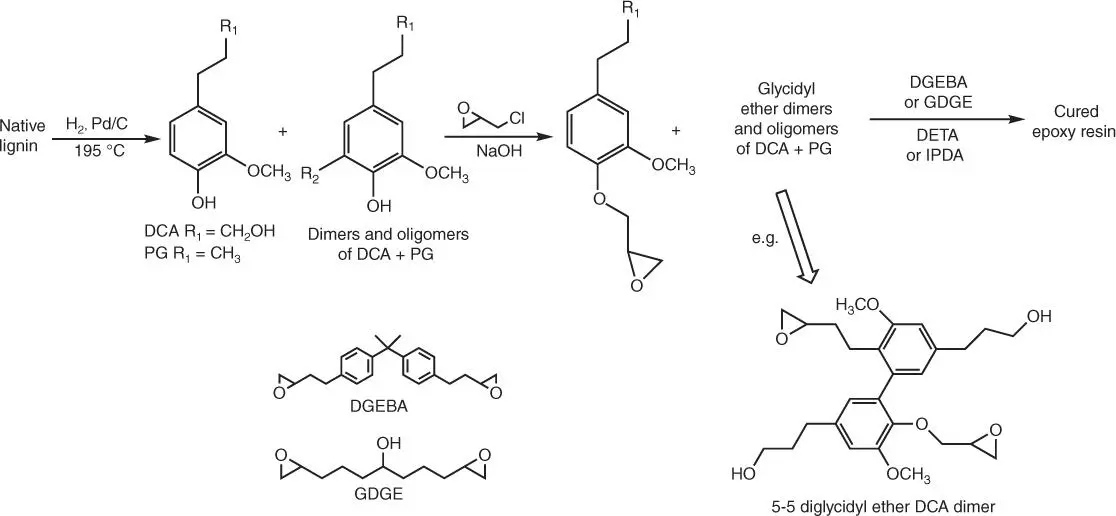
Figure 1.13 Cured epoxy resins from lignin hydrogenolysis products.
Table 1.4 Thermal decomposition of BPA‐based epoxy resin and the DGEBA/lignin‐based epoxy resin [72].
| Sample |
IDT (°C) |
T max(°C) |
Char 800(%) |
LOI |
|
|
Shoulder peak |
Main peak |
|
|
| DGEBA‐DDM |
370 |
— |
405 |
12.5 |
22.5 |
| 25% DKL‐DDM |
359 |
— |
397 |
18 |
24.7 |
| 50% DKL‐DDM |
330 |
— |
396 |
25 |
27.5 |
| 75% DKL‐DDM |
300 |
335 |
407 |
32 |
30.3 |
| 100% DKL‐DDM |
290 |
325 |
416 |
38 |
32.7 |
| 25% DOL‐DDM |
383 |
— |
404 |
17 |
24.3 |
| 50% DOL‐DDM |
352 |
— |
399 |
21 |
25.9 |
| 75% DOL‐DDM |
338 |
— |
398 |
24 |
26.7 |
| 100% DOL‐DDM |
335 |
— |
397 |
29 |
29.1 |
The lignin‐based epoxy material is characterized by comparable thermal and mechanical properties to those of BPA‐based epoxy resin (DER332) cured with the same bio‐based curing agent (the Diels–Alder adduct of methyl esters of eleostearic acid, a major tung oil fatty acid, and maleic anhydride [MMY]). The obtained bio‐based epoxy product might be applied as a modifier for asphalt applications in the same manner as petroleum‐based (and mostly BPA‐based) epoxy resins, which are currently used for asphalt modification to improve its temperature performance. The PDL epoxy asphalt, in the same way as DER332‐asphalt, exhibits significant improvement on the viscoelastic properties, especially at elevated temperatures.
The research on utilization of lignin derivatives toward the synthesis of the epoxy system is ongoing for several years; thus, there are numerous methods described in the literature. Among them, it is worth to mention the synthesis of epoxies by (i) direct epoxidation of the phenolic hydroxyl group in the technical lignin with epichlorohydrin and (ii) obtaining bisguaiacyl structure via the reaction using ketone compound and then the epoxidation ( Figure 1.15a) [66].
The other route of lignin utilization toward the synthesis of epoxy system is the cleavage of lignin intermolecular bond and creating the phenolic hydroxyl group in the molecule ( Figure 1.15c). The process is usually done by treating the Kraft lignin with acid (hydrochloric or sulfuric acid) and phenol derivatives. The obtained phenolic hydroxyl group is epoxidized with epichlorohydrin, resulting in the lignin‐based epoxy resin, which in the next step is cross‐linked using DETA or phthalic anhydride. The phenol derivative within the lignin structure might also be obtained on the course of the lignin phenolization with bisphenol A in the presence of hydrochloric acid and BF 3‐ethyl etherate as catalysts ( Figure 1.15b) [73]. The obtained product is soluble in organic solvent such as acetone because of the contribution of bisphenol A.
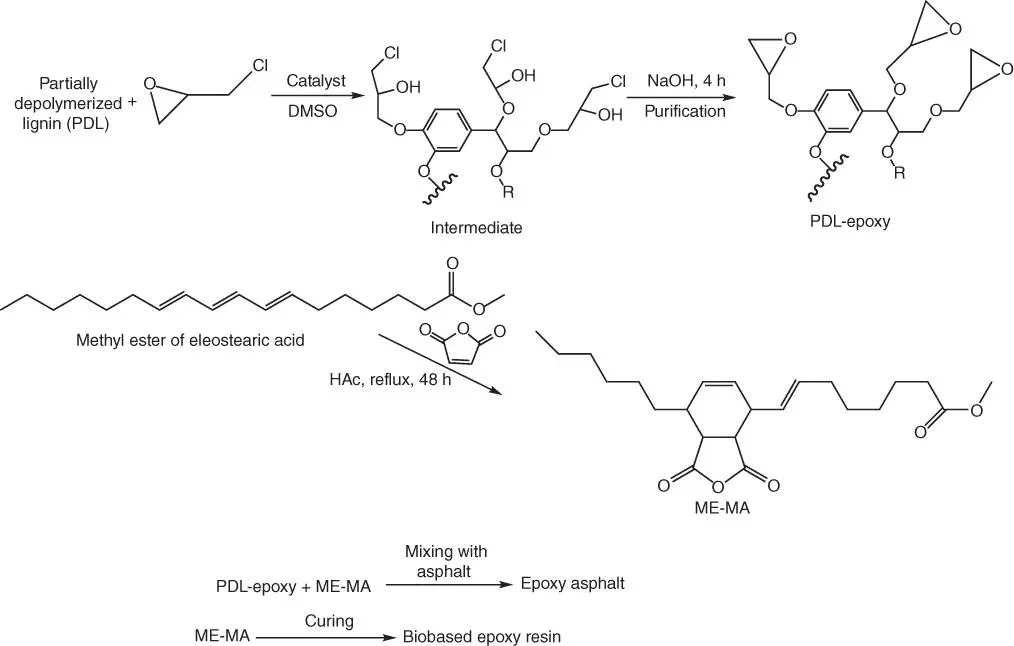
Figure 1.14 Synthesis of lignin‐based epoxy and epoxy asphalt.
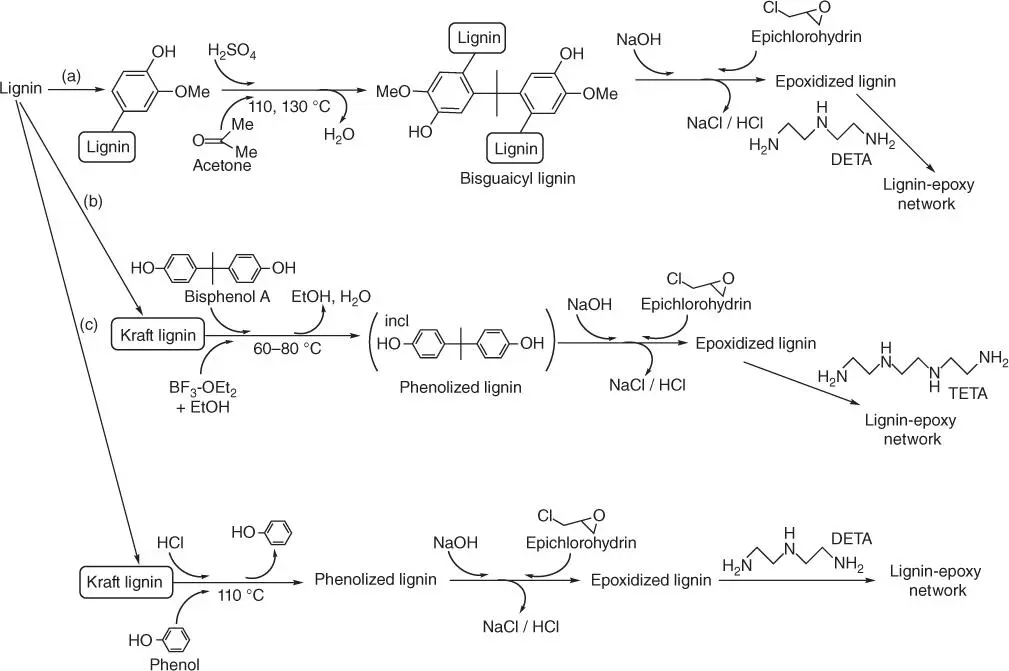
Figure 1.15 Schematic routes of lignin modification and crosslinking: (a) epoxidation with bisguaiacyl structure stage, (b) lignin' phenolization with bisphenol A and (c) direct epoxidation of the phenolic hydroxyl group in the technical lignin with epichlorohydrin.
Читать дальше
















