Stem Cells in Regenerative Endodontics
The potential utility of cord blood MSCs has not yet been fully realized in the relatively new field of regenerative endodontics. Since its development in 2004 by Banchs and Trope, regenerative endodontics has been employed as a root-preserving alternative to a root canal, utilized to eradicate pulp infections in immature permanent teeth, thus permitting further root development and preservation of teeth in patients who are still growing. 14 Liao et al 15revealed the presence of osteogenic MSCs in both inflamed pulp tissue and inflamed periapical tissues, and further investigation by Chrepa et al 16revealed that the evoked bleeding step demonstrated an increase in local accumulation of undifferentiated MSCs even in a mature tooth. Because of these revelations, the recommended American Association of Endodontists protocol was revised to use irrigants that are less toxic to stem cells and propose the use of platelet-rich plasma, platelet-rich fibrin, and autologous fibrin matrix in place of the simple blood clot.
However, there is no evidence in the literature of regeneration of the dentin pulp complex. The effect of noninflamed apical residual tissue in regeneration of pulp has been investigated recently in an animal model. Torabinejad et al 17conducted a study providing evidence that noninflamed apical residual pulp has the capability to regenerate the normal pulp. Given the discovery of the presence of stem cells involved in the restoration of the root, studies are now underway to assess if implantation of stem cells may in fact accelerate pulp tissue regeneration and healing and perhaps shorten the wait time. Tissue engineering permitted two strategies: (1) the direct implantation of freshly isolated stem cells with or without biodegradable scaffolds, and (2) implantation of preassembled tissue constructs containing in vitro cultured cells in the scaffold. 18Studies showed that the implantation of stem/progenitor cells isolated from a human root in a mouse model resulted in formation of a pulp-like tissue with a layer of dentin-like tissue along the canal wall. 19
Unfortunately, retrieval of autologous dental stem cells is difficult, especially in the common case where all other teeth are healthy. It is even more difficult to obtain one of the five subtypes of dental stem cells: (1) dental pulp stem cells (DPSCs), (2) stem cells from human exfoliated deciduous teeth (SHEDs), (3) stem cells from apical papilla (SCAPs), (4) periodontal ligament stem cells (PDLSCs), and (5) tooth germ progenitor cells (TGPCs). 20
As such, recent efforts have investigated the use of MSCs derived from other origins, such as human bone marrow–derived MSCs, which have potential for osteogenesis as a more multipotent stem cell than the differentiated dental stem cells. However, bone marrow–derived MSCs are associated with risks, mortality, and high cost of bone marrow harvesting and processing. Induced pluripotent stem cells have also been investigated, 21 , 22which studies show contribute to mesenchymal progenitors to create early cells in the osteogenic lineage. Unfortunately, the study by Sueyama et al 23using a rat model found that implanting MSCs alone showed incomplete dentin bridges, while coimplantation of MSCs with endothelial cells resulted in pulp healing with complete dentin bridge formation.
As such, a viable strategy to allow osteogenic regeneration involves the use of cord blood MSCs. This avoids the ethical concerns of embryonic stem cells and the morbidity of bone marrow acquisition while retaining the multipotency required for the regeneration of complex endodontic tissue. In 2018, Chen et al 24showed that MSCs derived from cord blood were able to achieve successful osteogenic and angiogenic properties, in addition to density, when cocultured with human umbilical vein endothelial cells. These cord blood MSCs had similar capabilities and performance as human bone marrow–derived MSCs and human embryonic stem cells. 24
Currently, the literature does not indicate any effect of cord blood stem cells used in combination with residual dental pulp tissue on the ability of a tooth to regenerate pulpal tissues. The authors propose that cord blood stem cells will enhance the ability of residual pulp tissue to regenerate.
Cord blood–derived and WJ-derived MSCs are an excellent option for therapeutic use because they are easily collected, readily available, and highly proliferative. They can also be used as allografts. Procedures used to isolate MSCs from these sources are described in the following sections.
Cord blood is collected from eligible donors at the time of delivery and transported to the processing facility on ice (2°C to 8°C) in a blood bag. Upon arrival, the blood is processed immediately under aseptic conditions (Fig 1-1a).
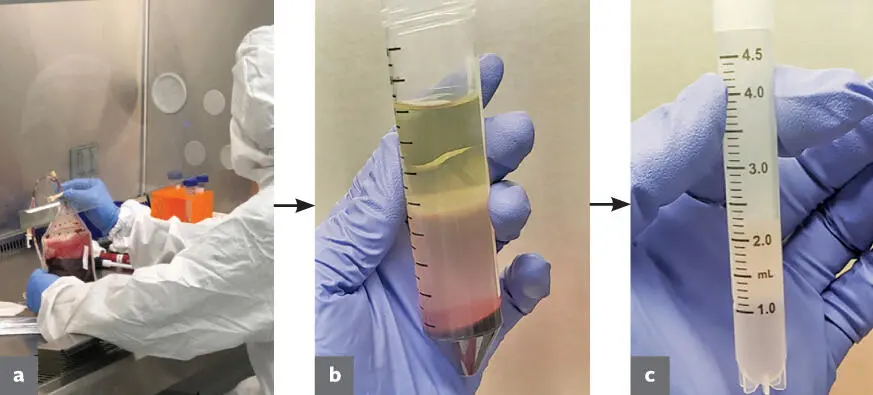
FIG 1-1 The different stages of cord blood processing. (a) Blood bag as it was received being processed under aseptic conditions. (b) Conical tube after centrifugation. Note plasma layer (top) , buffy coat layer (middle) , and red blood cells (bottom) . (c) PBMCs suspended in Stem-Cellbanker ready for storage in a cryovial.
The blood bag is first drained into conical tubes and centrifuged at 1500 × g to separate components. This results in distinct layers in the conical tube, with plasma rising to the top and red blood cells forming a pellet at the bottom of the tube. There is also a distinct buffy coat layer below the plasma that contains the cells of interest. The buffy coat layer is then collected and diluted with a phosphate-buffered saline (PBS) solution before undergoing density gradient centrifugation. The buffy coat and PBS mixture is added to Ficoll-Paque (GE Healthcare) density gradient media and then centrifuged at 409g. This results in an isolation of peripheral blood mononucleated cells (PBMCs) in the resulting buffy coat (Fig 1-1b).
The PBMCs are then collected and diluted once again with PBS before being centrifuged at 409 × g. This wash step is repeated until the resulting supernatant is no longer cloudy or hazy, and the resulting pellet is then resuspended in Stem-Cellbanker (Amsbio). Cell count, viability, and surface marker profile are then tested using flow cytometry. This information is used to aliquot the appropriate cell number into cryovials, and the cells are immediately stored at –80°C (Fig 1-1c).
Umbilical cords are collected from eligible donors at the time of delivery and transported to the processing facility on ice (2°C to 8°C) in Dulbecco’s Modified Eagle Media (DMEM). Cords are processed immediately under aseptic conditions, and MSCs are collected for culture according to the procedure described below.
To start a primary explant culture, a roughly 1 × 1–cm segment of cord is obtained using sterile forceps and scalpel. This segment is dissected to remove blood vessels and isolate the WJ. The resulting segments of WJ are then added to 10 mL of a collagenase-DMEM solution and incubated at 37°C for 4 hours. The collagenase-DMEM solution is prepared to a strength of 300 collagenase degrading units (CDU) per mL. Digested pieces of tissue are then collected using sterile forceps and transferred to a T-25 flask containing 10 mL of MSC-Brew Xeno-Free Media (Miltenyi Biotec). Cultures are then incubated for 72 hours at 37°C, at which point the media is replaced and the pieces of tissue are removed from the flask.
Читать дальше













