Each layer of carbon nanofiber or nanotubes, contains a set of carbon atoms arranged in one plane. In x-y direction, each carbon is attached with σ-bonds. Bottom layers of carbons (in z direction) are also arranged in the same fashion, such that each carbon shares its electron with bottom layers of carbon by either σ-bonds or π-bonds as the case may be ( Figure 1.7a). However, electrons of carbon atoms present in the top layer are not satisfied with its electron due to the absence of any carbon atoms above its layer. These carbon atoms are dissatisfied with electrons. As a result, these carbon atoms would either behave as positively charged (lost its electron) or negatively charged (contains more than its normal electron). However, these distributions of charges would be such that overall the carbon tube will behave like a neutral particle. These unsatisfied carbon atoms are more reactive as compared to carbon atoms present in the layers below the top layer. These carbon atoms are said to possess active sites which are designated as a surface state. The presence of surface state depends upon the types of graphene layers present on the top layers of carbon tube. Its broken graphene layers will show a large number of surface states as well as surface areas. It is for this reason that carbon nanofibers are more reactive as compared to carbon nanotubes.
1.3 Synthesis of Carbon Nanofiber (CNF)
Catalytic chemical vapor deposition (CCVD) or simply CVD is the foremost commercial technique for the fabrication of CNF as well as CNT. This is a batch process. This technique also produces CMF known as vapor-grown carbon nanofiber (VGCNF). In this type of process, precursor near its boiling temperature is converted into gas-phase molecules and is transported by some carrier gas to decompose at high temperatures in the presence of a transition metal catalyst kept on a substrate. A subsequent growth of the fiber around the catalyst particles is achieved. The nanofiber diameter depends on the catalyst size. There can be different designs of a CVD unit.
1.3.1 Chemical Vapor Deposition (CVD) Method
A typical CVD setup is shown in Figure 1.8. A long quartz tube (C) is inserted into two ceramic tubes (D). Electrical heating wire is wrapped over two ceramic tubes (D). These two ceramic tubes are arranged to maintain two different temperatures (A1 and A2). In the quartz tube (C) two quartz boats (B1 and B2) are kept. In boat B1 a known amount of precursor which is to be pyrolyzed is kept. In boat B2 catalyst powder is spread over the boat. Two gas cylinders (K and H) are connected to the long quartz tube (C) via two controllers (E) and flow meter (F). The outlet of tube C is connected to two bubblers (G). Temperature of A1 is maintained at a value which can boil the precursor kept in quartz boat B1, which is normally kept at around 400 °C. Temperature of furnace A2 is maintained at a suitable temperature where the precursor gas can be decomposed in the presence of the catalyst to get the CNF. Before setting the temperatures of two furnaces, inert gas like argon gas (H) is purged through the quartz tube (C) to remove the presence of oxygen. The temperature of furnace A2 is set to a required temperature (which could be in the range of 600–1000 °C). When the required temperature has attained the temperature of the furnace, A1 is set to boiling temperature of the precursor. During this process the flow of carrier gas is maintained which helps to carry the vapor of precursor to the furnace A2 for completing the process of pyrolysis. Undecomposed gas and carrier gas are allowed to escape to atmosphere through the bubblers (G). At the end of pyrolysis CNF is collected from the furnace (A2).
It is possible to control the length of carbon nanofibers by controlling the rate of gas flow in the quartz tube (C). In addition, direction of fiber growth can also be controlled by adjusting the direction of gas flow. If adjusted properly, the variable parameters of the process can yield CNF with submicrometer diameters and lengths of a few to 100 μm.
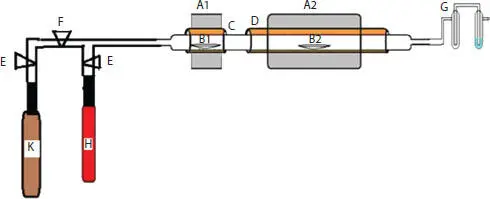
Figure 1.8 A sketch of a typical CVD unit. A1 and A2 are two furnaces operating at two different temperatures. B1 and B2 are two quartz boats used for keeping precursor and catalyst respectively. CNF is grown on the boat B2 as well as over the entire area covering the furnace A2, C is the quartz tube in which growth of CNF takes place, D is ceramic tube over which heating wire is wrapped to create required temperature, E and F are gas valves and flow meter to regulate flow rate of gas. G is a gas bubbler to prevent backflow of gas into the quartz tube and allow the escape of unreacted gas into the atmosphere, H is the gas cylinder containing the carrier gas and K is precursor gas like methane or acetylene.
Precursor for synthesizing CNF can be several types of hydrocarbons such as natural oils like mustard oil, pure fatty acids (liquid or solid), camphor, terpene oil, acetylene, ethylene, methane, natural gas, benzene, etc. Introduction of carbon monoxide (CO) into the gas flow can increase the carbon yield, hence yields a greater amount of CNF. It is also possible to use plant seed materials like mustard seed, stem of any plant, plant leaves, bamboo, rice straw, bagasse, etc. It is also possible to use some gasses directly like methane, acetylene, etc. When using solid materials like plant leaves, there is no need to use the furnace ( Figure 1.8A1). These precursors can be kept directly in the boat ( Figure 1.8B2) along with any catalyst (if needed). If gases like methane are to be used as a precursor then there is also no need to use the furnace ( Figure 1.8A1). Gas from the cylinder ( Figure 1.8K) can be sent to the furnace ( Figure 1.8A2) where suitable catalyst is kept in the boat. The precursor gas can either be directly fed into the unit or is mixed with carrier gas in the required proportion.
1.3.3 Use of Catalyst in the Synthesis of CNF
There are variations in the type of catalyst used for the synthesis of CNF. There are some catalysts which are solid like ferrocene, cobaltocene, etc. These catalysts vaporize in the temperature range of 300 to 400 °C. Such catalysts are kept in a boat ( Figure 1.8B1) in the furnace ( Figure 1.8A1) along with the precursor like oil. The vapor of oil and the vapor of catalyst like ferrocene are carried with carrier gas to the furnace ( Figure 1.8A2). Catalyst decomposes into its metal oxide and in-situ reaction takes place with the vapor of oil to produce CNF. These CNFs are collected in the boat ( Figure 1.8B2).
Catalyst can be used either in the form of powder or as a thin film deposited on a substrate like quartz glass, alumina plate, or as a metal plate like nickel, iron metal, etc. Normally, after scratching a metal plate with diamond powder it is cleaned with water and dried with acetone, which is also used as a catalyst. Scratching the plate creates more roughness of the metal, which produces more surface states and surface area over which the reaction of vapor of precursor reacts more favorably with catalyst. The preferred size of catalyst particles should be in the range of 5–25 nm in diameter. Some examples of the role of different types of catalysts and their effect on the carbon nanomaterials produced are shown in Figure 1.4.
There is no hard and fast rule to suggest which type of catalyst will produce better CNF. Better CNF means the value of its diameter, length, surface area and surface states. Selection of catalyst and its type depends upon the type of precursor and size of catalyst powder, which can only be decided by carrying out some specific experiments. Powder or thin films of Fe/Ni, Ni, Co, Mn, Cu, V, Cr, Mo, Pd, MgO, and Al 2O 3are used as catalyst. But most popular catalysts are powder or thin film of organometallic compounds of iron or nickel or cobalt. Details of the use of different catalysts are presented in Chapter 3of this book.
Читать дальше



![Carbon - Посох с проблемами [калибрятина, куски]](/books/397599/carbon-posoh-s-problemami-kalibryatina-kuski-thumb.webp)

![Carbon - Ключ от магии, или Нимфа по вызову [СИ]](/books/417919/carbon-klyuch-ot-magii-ili-nimfa-po-vyzovu-si-thumb.webp)
![Carbon - Девушка с Косой [СИ]](/books/427728/carbon-devushka-s-kosoj-si-thumb.webp)






