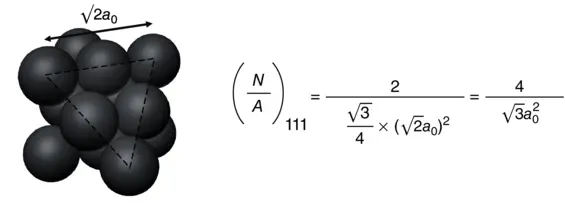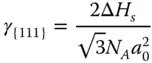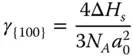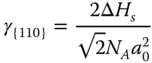The excess energy at the surface of a material compared with the bulk is defined as the surface energy. Without facets engineering, the most stable free surface will occupy the greatest portion of the crystal surface as it attempts to minimize the total surface energy in a given growth condition. According to the Gibbs–Wulff theorem, the facets with higher surface energies always grow faster and end up with a tiny fraction or even vanish [20]. The Wulff construction method predicts the shape of a crystal with the lowest surface energy, and the most stable form is called the equilibrium shape of a crystal. Owing to the anisotropic surface energy of crystal facets, the final shape of a single crystal is usually enclosed with the facets of lowest surface energy and smallest surface area in a given volume. For example, for a metal crystal that contains N Aatoms and each bulk (i.e., nonsurface) atom that is defined by a coordination number of CN, there will be ( N A× CN)/2 bonds in the crystal. As such, the energy of each bond can be calculated as
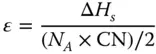
where ΔH sis the molar enthalpy of the metal sublimation.
A face‐centered cubic (fcc) structured metal crystal typically has three low‐index facets, i.e. {111}, {100}, and {110} facets. While each bulk atom has a CN of 12 (6 around the side perimeter of the atom and 3 each on top and bottom), depending on the direction of the surface cut, which, in turn, dictates the exposed facets, the atom on terminated surface would lose a certain number of atoms and result in dangling bonds.
As shown in Figure 2.1a, the CN of the atoms at the {111} surface is 9, which means 3 bonds are broken. Therefore, the energy required per atom to form the {111} surface can be calculated as
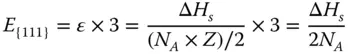
Then, the surface energy of {111} facet γ {111}can be calculated as follows:
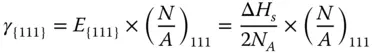
where 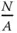 is the number of surface atoms per area.
is the number of surface atoms per area.
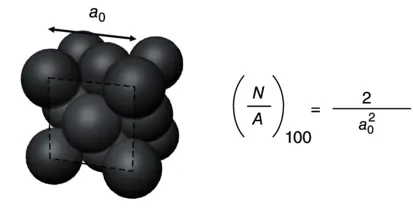
Similarly, the CN of the atoms at the {100} surface is 8, which means 4 bonds are broken ( Figure 2.1b). Therefore, the energy required per atom to form the {100} surface can be calculated as
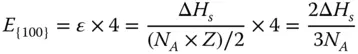
Then, the surface energy of {100} facet γ {100}can be calculated as follows:
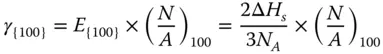
The {110} surface contains two layers of atoms ( Figure 2.1c). The CN of the first‐layer atoms is 7, that is, 5 bonds are broken. And the CN of the second atoms is 11, that is, 1 bond is broken. Therefore, the energy required per atom at the first layer and second layer can be calculated as
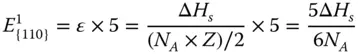
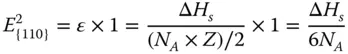
The surface energy of {110} facet γ {110}can be calculated as follows:
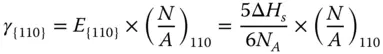
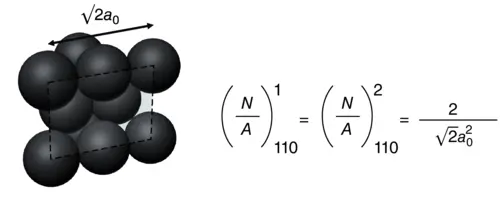
If a 0is the lattice constant,  of each surface can be obtained as
of each surface can be obtained as
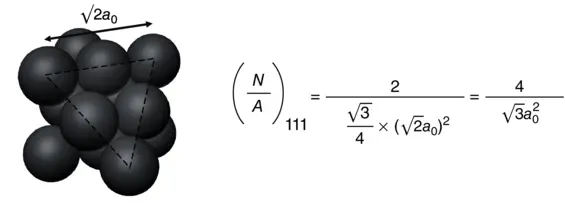
The surface energy of each facet is
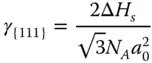
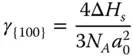
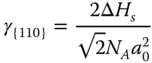
where γ {110}> γ {100}> γ {111}.
Considering the surface energy order is {110} > {100} > {111}, the final crystal has a tendency to form an octahedron‐shaped crystal that is dominated with {111} facet, rather than a cube enclosed by {100} facet (as shown in Figure 2.1). However, the octahedral shape has a larger surface area than the cube of the same volume. As a consequence, the shape turns to be a truncated octahedron with a mix of {100} and {111} facets [1]. Another example is anatase TiO 2. According to the Wulff construction and surface energy calculation, the equilibrium shape of anatase TiO 2crystal (as shown in Figure 2.1) is a slightly truncated bipyramid enclosed with 94% {101} facet and 6% {001} facet [21], although the order of the surface energy of low‐index facets is {001} (0.90 J/m 2) > {010} (0.53 J/m 2) > {101} (0.44 J/m 2) [22].
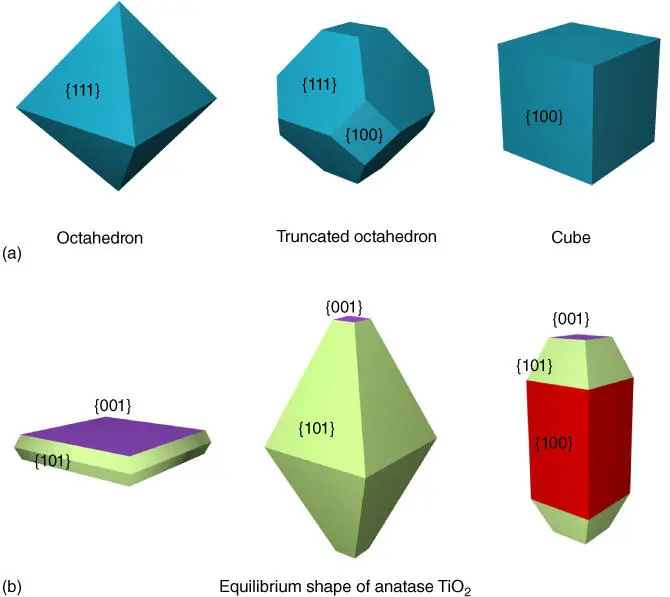
Figure 2.1 (a) Octahedron, truncated octahedron, and cube with the same volume. (b) The equilibrium shape of anatase TiO 2(middle) and two variants.
In practice, the product often shows a different shape from that predicted by equilibrium. The possible reasons may be that (i) the equilibrium condition was not fully satisfied during the synthesis, and/or (ii) the anisotropic surface energies of different facets were interfered by impurities or other factors. In other words, this allows the manipulation of the nucleation and crystal growth by intentional addition of impurities and tuning of synthesis conditions to achieve product particles with desired shape and exposed facets. This is the core concept of facets engineering.
Selectively controlling the nucleation and anisotropic growth rate is known as the bottom‐up route. The most common method is to use a selective capping agent to reduce the surface energies of the adsorbed facets, or to change the order of surface energies of different facets, or to terminate the crystal growth of a selective facet. Figure 2.2indicates how the solvents and capping agents can be used to tune the morphologies during the crystal growth [23]. The capping agents can be atomic or molecular species originating from a gas or liquid environment. As early as in 1986, it was found that H 2S could cause drastic morphological changes of Pt nanocrystals [24]. Pt{100} facet had a stronger interaction with sulfur than Pt{111} facet, resulting in the formation of Pt nanocubes rather than Pt nanospheres. More capping agents are generally used in the solution‐phase synthesis. For example, inorganic species such as bromides and organic species such as poly(vinylpyrrolidone) (PVP) are very popular for tailoring both metal [25–27] and semiconductor crystals [28–30].
Читать дальше

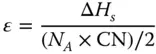
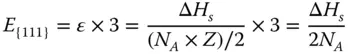
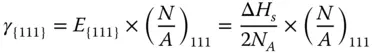
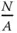 is the number of surface atoms per area.
is the number of surface atoms per area.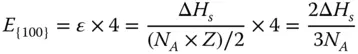
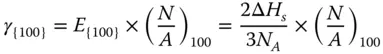
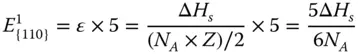
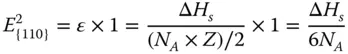
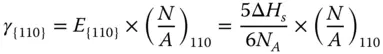
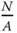 of each surface can be obtained as
of each surface can be obtained as