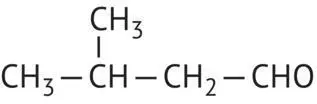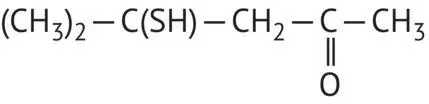2.5.4 Esters of Chemical Origin
The formation of esters continues throughout the aging process, thanks to the presence of nonvolatile acids in wine together with large quantities of ethanol. Research into esterification mechanisms in wine (Ribéreau‐Gayon et al ., 1982) showed that, under normal cellar conditions, none of the acids ever reach the equilibrium predicted in theory. The ester content represents approximately 30% of the theoretical limit after 1 year, 50% after 2 or 3 years, and 80% after 50 years. The total ester concentration (regardless of its origins) is governed by the wine's composition and age. It varies from 2 or 3 mEq/l in young wines up to 9 or 10 mEq/l in old wines, in which approximately 10% of the acids are esterified.
Mono‐acids react with ethanol to form only neutral esters, whereas di‐acids may produce one neutral and one acidic ester (e.g. ethyl tartrate and ethyl hydrogen tartrate). On average, wine contains approximately the same quantity of neutral and acidic esters. The latter contribute to wine acidity.
Ethyl lactate is a special case. Its formation is linked to malolactic fermentation, with the possible involvement of an esterase of bacterial origin. However, concentrations of ethyl lactate also increase throughout aging via chemical reactions. In Champagne wines that have completed malolactic fermentation, the ethyl lactate concentration has been observed to increase to a maximum of 2 g/l after two years and then decrease during further aging on the lees. According to Arctander (1969), ethyl lactate has an odor reminiscent of butter or even sour milk. Other authors think that the odor of ethyl lactate has been confused with that of other aroma compounds.
2.6 Miscellaneous Compounds
Among the other volatile products likely to contribute to wine aroma are volatile phenols and sulfur derivatives. The latter are responsible for off‐odors whose causes and consequences are now well known and are described elsewhere in this volume (Sections 8.4 and 8.6). There are also several compounds that contribute to the varietal aromas of different grape varieties, e.g. terpenes in Muscats (Section 7.2.1). These compounds are also discussed at length in Chapter 7.
This section is thus exclusively devoted to carbonyl compounds, lactones, and acetals.
2.6.1 Carbonyl Compounds (Aldehydes and Ketones)
Acetaldehyde is the most important of these compounds (Table 2.7). The many ways it can be produced, its high reactivity, its rapid binding with sulfur dioxide at low temperatures, and its organoleptic properties make acetaldehyde a very important component of wine. The presence of acetaldehyde, produced by the oxidation of ethanol, is closely linked to oxidation–reduction phenomena. It is involved in the alcoholic fermentation mechanism. Furthermore, acetaldehyde plays a role in the color changes occurring in red wines during aging by facilitating the copolymerization of phenols (anthocyanins and catechins) (Section 6.3.10).
In wine preserved with regular, light sulfiting, the bound form of acetaldehyde and sulfite (CH 3−CHOH−SO 3H), stable in an acidic medium, is the most prevalent form (Volume 1, Section 8.4.1). When grapes have been heavily sulfited, the acetaldehyde concentration increases and may exceed 100 mg/l in the bound form with sulfite. This sulfite binding of acetaldehyde protects yeast from the antiseptic effects of SO 2.
Wines containing excess acetaldehyde as compared with the quantity of SO 2, i.e. free (unbound) acetaldehyde, are described as “flat” (Section 8.2.3). A slight trace of free acetaldehyde is sufficient to produce a characteristic odor, reminiscent of oxidized apple. This problem disappears rapidly if a little SO 2is added, as it binds with the free acetaldehyde. This is one of the reasons for sulfiting barrels during racking (Section 10.3.3).
A few other aldehydes are present in wine in trace amounts (Table 2.7). These include Strecker aldehydes, associated with the oxidative aging of white wines, such as methional, formed from methionine and phenylacetaldehyde (from phenylalanine) (Section 8.6). The neutralizing effect of sulfur dioxide on the fruitiness of certain white wines is due to the fact that it binds with the aldehyde fraction.
Aldehydes in the aromatic series are also present in wine. The most significant of these is vanillin, which is associated with barrel aging and has a distinctive vanilla aroma.
Grapes apparently contain few aldehydes. Hexenal and hexenol have, however, been identified as contributing to the vegetal and grassy odors of C6 compounds ( Section 2.2.3).
Several molecules with ketone functions have been identified, including propanone, butanone, and pentanone. As previously mentioned, the most important of these are acetoin (acetyl methyl carbinol) and diacetyl ( Section 2.3.2).
Compounds with more than one aldehyde or ketone function have been identified: glyoxal and methyl glyoxal (Volume 1, Section 8.4.4).
Finally, a mercaptopentanone (4‐MMP) has been identified, along with various other thiols, as one of the specific components of Sauvignon Blanc aroma (Section 7.5.1).
TABLE 2.7Aldehydes and Ketones in Wine
| Formula |
Name |
Boiling point (°C) |
Comments |
| H−CHO |
Methanal |
21 |
Formaldehyde |
| CH 3−CHO |
Ethanal |
21 |
Acetaldehyde. In bound form with SO 2. Only oxidized wines ( Rancio , Sherry, etc.) contain free acetaldehyde |
| CH 3−CH 2−CHO |
Propanal |
49 |
|
| CH 3−CH 2−CH 2−CHO |
Butanal |
76 |
Butyraldehyde |
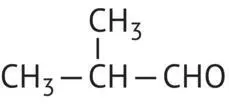 |
2‐Methyl‐propanal |
92 |
Isobutyraldehyde |
| CH 3−CH 2−CH 2−CH 2−CHO |
Pentanal |
102 |
Valeraldehyde |
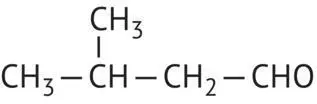 |
3‐Methylbutanal |
92 |
Isovaleraldehyde |
| CH 3−CH 2−CH 2−CH 2−CH 2−CHO |
Hexanal |
128 |
Caproaldehyde |
| CH 3−CH 2−CH 2−CH=CH−CHO |
2‐Hexenal |
|
Only presentin grapes |
| CH 3−(CH 2) 5−CHO |
Heptanal |
155 |
Enanthaldehyde |
| CH 3−(CH 2) 6−CHO |
Octanal |
167 |
Caprylaldehyde |
| CH 3−(CH 2) 7−CHO |
Nonanal |
185 |
Pelargonaldehyde |
| CH 3−(CH 2) 8−CHO |
Decanal |
208 |
Caprinaldehyde |
| CH 3−(CH 2) 10−CHO |
Dodecanal |
|
Lauraldehyde |
| CH 3−CO−CH 3 |
Propanone |
56 |
Acetone |
| CH 3−CH 2−CO−CH 3 |
Butanone |
80 |
Methyl ethyl ketone |
| CH 3−CH 2−CH 2−CO−CH 3 |
2‐Pentanone |
102 |
|
| CH 3−CHOH−CO−CH 3 |
Acetyl methyl carbinol |
143 |
Acetoin |
| CH 3−CO−CO−CH 3 |
Diacetyl |
87 |
|
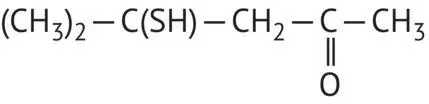 |
4‐Mercapto‐4‐methyl‐2‐pentanone |
|
Boxwood aroma of Sauvignon Blanc wines |
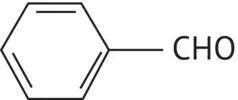 |
Benzaldehyde |
178 |
|
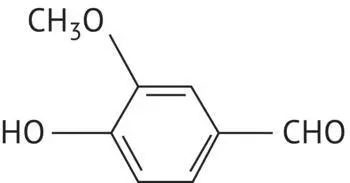 |
Vanillin |
285 |
|
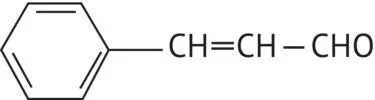 |
Cinnamaldehyde |
253 |
|
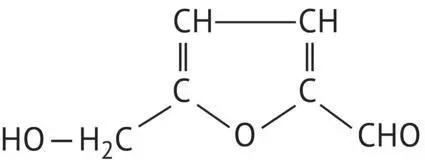 |
Hydroxymethylfurfural |
|
Grape juice or wine subjected to heat treatment |
Читать дальше