29 Moine‐Ledoux V. and Dubourdieu D. (2002) Bull. OIV, 75, 857.
30 Moine‐Ledoux V., Perrin A., Paladin I. and Dubourdieu D. (1997) Œno One, 31, 1, 23.
31 Müller‐Späth H. (1979) Rev. Fr. Œnol., 41, 47.
32 Ordonneau C. (1891) Bull. Soc. Chim. Fr., 261.
33 Pellerin P., Waters E., Brillouet J.‐M. and Moutounet M. (1994) Œno One, 23, 3, 213.
34 Peynaud E. and Blouin J. (1996) Le goût du vin. Dunod, Paris.
35 Peynaud E. and Guimberteau G. (1961) Ann. Falsif. Fraud., 53, 567.
36 Postel W. (1983) Bull. OIV, 629–630, 554.
37 Prelog V. (1953) Helv. Chim. Acta, 36, 308.
38 Ribéreau‐Gayon J., Peynaud E., Sudraud P. and Ribéreau‐Gayon P. (1977) Sciences et Techniques du Vin, Vol. IV: Clarification et Stabilization. Dunod, Paris.
39 Ribéreau‐Gayon J., Peynaud E., Sudraud P. and Ribéreau‐Gayon P. (1982) Sciences et Techniques du Vin, Vol. I: Analyse et Contrôle du Vin, 2nd edition. Dunod, Paris.
40 Robillard B., Baboual S. and Duteurtre B. (1994) Rev. Fr. Œnol., 145, 19.
41 Usseglio‐Tomasset L. (1989) Chimie Œnologique. Tec et Doc, Lavoisier, Paris.
42 Vallée D. (1995) Œno One, 29, 143.
43 Vergnes P. (1940) Coefficient tampon des vins, Thèse de Doctorat, Université de Montpellier.
44 Vialatte C. and Thomas J.‐C. (1982) Rev. Fr. Œnol., 87, 37.
45 Wurdig G. and Muller T. (1980) Die Weinwirtschaft, 116, 720.
46 Wurdig G., Muller T. and Fiedrich G. (1982) Bull. OIV, 613, 220.
CHAPTER 2 Alcohols and Other Volatile Compounds
1 2.1Ethanol
2 2.2Other Simple Alcohols
3 2.3Polyols
4 2.4Aliphatic Fatty Acids
5 2.5Esters
6 2.6Miscellaneous Compounds
Besides water, ethanol (ethyl alcohol) is the most plentiful compound in wine. A wine's strength is expressed in terms of alcohol content or the percentage of alcohol by volume. As ethanol has a specific gravity of 0.79, a wine with an alcohol content of 10% vol. contains 79 g/l of ethanol by weight. The alcohol content of wine, expressed in terms of density, is generally 100 g/l (12.6% vol.), although it may exceptionally be as high as 136 g/l (e.g. an alcohol content of 16% vol.).
Due to the low specific gravity of ethanol, dry wines, containing negligible amounts of residual sugar, have specific gravities below that of water (1.00), ranging from 0.91 to 0.94. This value decreases as the alcohol content increases.
Ethanol in wine is mainly produced by the alcoholic fermentation of sugar in must. However, grape cells are also capable of forming small quantities, mainly under anaerobic conditions (carbonic maceration; see Volume 1, Section 12.9.3). The appearance of traces of ethanol in grapes results from alcohol dehydrogenase activity, which acts as a marker for ripeness.
As approximately 16–18 g/l of sugar is required to produce 1% vol. of ethanol during alcoholic fermentation, grape must has to contain 180, 226, and 288 g/l of sugar to produce wines with 10, 12.6, and 16% ethanol by volume, respectively. The latter is considered to be the maximum ethanol content at which yeast can survive, although under certain laboratory conditions, some strains have been found capable of resisting up to 18% vol. Some types of wine may, of course, have even higher alcohol contents, but this results from the addition of ethanol.
Many consumers place excessive importance on alcohol content as an essential quality factor. Most countries legally require the alcohol content to be displayed on wine labels. Historically, wines were marketed on the basis of alcohol content.
In temperate climates, the natural alcohol content depends directly on grape ripeness. Wines only have a high alcohol level in years when the weather is particularly good, and if vineyard conditions and exposure to the sun are favorable. Great vintages are often years when wines reach high alcohol contents. It would, however, be ridiculous to suppose that alcohol is the only quality factor. Some excellent Médoc wines have an alcohol content of around 10% vol., while other high‐alcohol wines are heavy, undistinguished, and unattractive.
In chemical terms, ethanol is a primary alcohol ( Figure 2.1), i.e. its C1 is tetrahedrally hybridized sp 3and carries two hydrogen atoms twinned with the hydroxyl radical (─OH). Alcohol functions should not be confused with enol functions
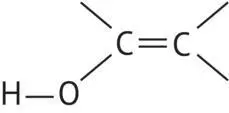
or phenol functions (OH bonded to a carbon in a benzene cycle). In both of these cases, carbon, the hydroxyl radical carrier, is hybridized sp 2and the acid character of the hydroxyl proton is much more pronounced. Consequently, the function is more easily salifiable.
In ethanol produced by fermentation, some hydrogen atoms on C1 and C2 in certain molecules are replaced by deuterium ( 2H or D), an isotope of hydrogen. These molecules are present in very small amounts, and the exact proportion depends on the origin of the sugar that was fermented (grapes, beets, and/or sugarcane), as well as on the growing region. A method for controlling the addition of sugar to must (chaptalization) and detecting fraud has been developed on the basis of this property (Martin and Brun, 1987). This method has been officially recognized by the International Organisation of Wine and Vine (OIV).
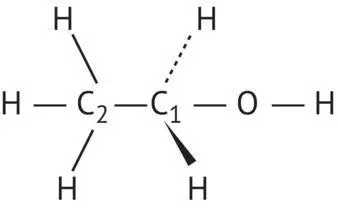
FIGURE 2.1 Structure of ethanol and definition of the alcohol function.
Ethanol's affinity for water and its solubility, by forming hydrogen bonds, makes it a powerful dehydrant. This property is useful in flocculating hydrophilic colloids, proteins, and polysaccharides. It also gives ethanol disinfectant properties that are particularly valuable in aging wine. The combination of ethanol and acidity makes it possible to keep wine for a long time without any noticeable spoilage. The addition of ethanol to stabilize certain wines is a long‐standing winemaking tradition (Port, vins doux naturels ). However, ethanol is toxic for humans, affecting the nerve cells and liver.
Ethanol's solvent properties are also useful for dissolving phenolic compounds from grape skins during red winemaking. This property is involved in solubilizing certain aroma molecules and certainly contributes to the expression of aromas in wine.
Ethanol has all the chemical properties of an alcohol function. In particular, it esterifies with tartaric, malic, and lactic acids ( Section 2.5.3). Ethyl acetate gives wine an unpleasant odor and is a sign of bacterial spoilage ( Section 2.5.1).
Ethanol may also react with hydrogen sulfide, produced by fermenting yeast or resulting from the residues of some vineyard treatment products (Section 8.6.3). This substitution reaction generates ethanethiol ( Figure 2.2), which has a very unpleasant smell. As this compound is much less volatile than H 2S, it is more difficult to eliminate. It is therefore advisable to rack wines as soon as alcoholic fermentation is completed and again immediately after malolactic fermentation, since hydrogen sulfide may also be produced by lactic acid bacteria (Volume 1, Section 13.9.1). Furthermore, the oxidation–reduction equilibrium may also cause ethanethiol to form diethyl disulfide ( Figure 2.3). This compound is even less volatile and has a very unpleasant smell, which is negatively perceived during wine tasting.

FIGURE 2.2 Reaction between hydrogen sulfide and ethanol.
Читать дальше















