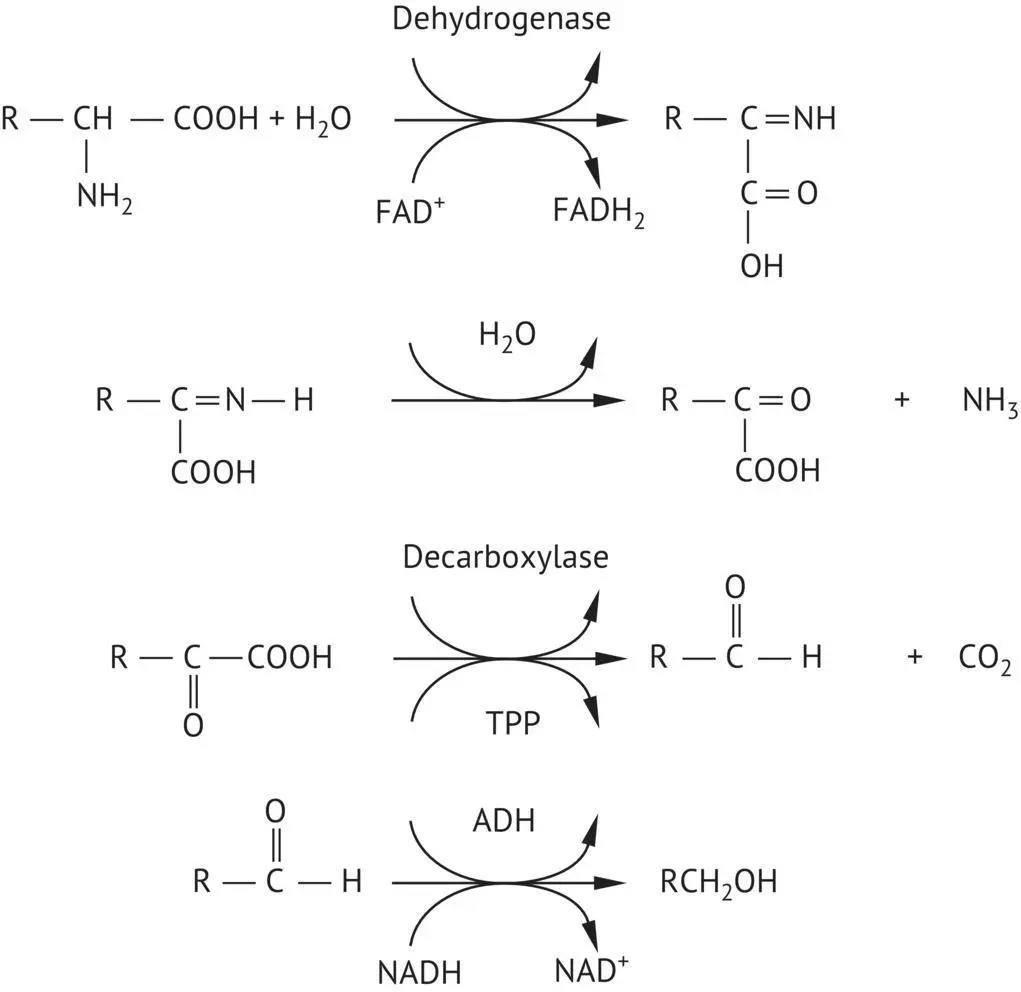
FIGURE 2.4 Biosynthesis of higher alcohols, according to Ehrlich.
Higher alcohols are found in brandies after distilling and contribute to their specific characters. Distillation techniques have a major impact on their overall concentration.
2.2.3 Miscellaneous Alcohols
These molecules originate from grapes and are found in wines. One group consists of C6 alcohols, hexanols and hexenols from plant tissues, giving the herbaceous/grassy aromas so characteristic of wines made from unripe grapes (Volume 1, Sections 11.6.2 and 13.3.4).
Another of these compounds is 1‐octen‐3‐ol, with an odor reminiscent of mushrooms. Its presence in wine is due to the action of Botrytis cinerea on grapes.
Finally, terpene alcohols, the main components in the distinctive Muscat aroma, are described in full elsewhere (Section 7.2.1).
Polyols are characterized by the presence of several hydroxyl radicals in the same linear or cyclic molecule. In general, an accumulation of hydroxyl radicals in a compound raises the boiling point considerably (Table 2.2) due to the large number of hydrogen bonds, as well as increasing its viscosity and anti‐freeze properties. Parallel increases are observed in solubility and sweetness. Sugars are good examples of polyols.
TABLE 2.2Impact of the Number of Hydroxyl Groups on the Boiling Point of Alcohols
|
Alcohols and polyols |
Boiling point(°C) |
| Ethanol |
CH 3−CH 2OH |
78 |
| Ethyleneglycol |
CH 2OH−CH 2OH |
198 |
| Glycerol |
CH 2OH−CHOH−CH 2OH |
290 |
2.3.1 C3 Polyol: Glycerol
Besides water and ethanol, glycerol (Table 2.3) is probably the chemical compound with the highest concentration in wine. It is the most important by‐product of alcoholic fermentation. The minimum glycerol concentration in wine is 5 g/l, but it may reach values as high as 15–20 g/l, depending on fermentation conditions (especially must sulfiting levels). Musts from grapes affected by noble rot already contain a few grams of glycerol, which is added to the quantity produced by fermentation.
Glycerol is formed by yeast at the beginning of the fermentation process. It is generally considered to be produced “with the first 50 grams of sugars fermented.” This corresponds to the start of the glyceropyruvic fermentation. The only way for yeast to ensure the reoxidation of the NADH coenzyme + H +is by reducing dihydroxyacetone to glycerol. At this stage, the acetaldehyde level is too low for this reoxidation to occur while producing ethanol. When must is treated with high doses of SO 2, this molecule binds with acetaldehyde, thus increasing the glyceropyruvic fermentation rate and the amount of glycerol formed.
TABLE 2.3Concentrations of Polyols Found in Wines (Ribéreau‐Gayon et al., 1982)
|
Formula |
Concentrations(mg/l) |
| Glycerol |
CH 2OH−CHOH−CH 2OH |
5,000–20,000 |
| 2,3‐Butanediol |
CH 3−CHOH−CHOH−CH 3 |
330–1,350 |
| Erythritol |
CH 2OH−(CHOH) 2−CH 2OH |
30–200 |
| Arabitol |
CH 2OH−(CHOH) 3−CH 2OH |
25–350 |
| Mannitol |
CH 2OH−(CHOH) 4−CH 2OH |
90–750 |
| Sorbitol |
CH 2OH−(CHOH) 4−CH 2OH |
30–300 |
| meso ‐Inositol |
(CHOH) 6 |
220–730 |
Glycerol in wine may act as a carbohydrate nutrient for the growth of various microorganisms, e.g. the flor yeast in Sherry production (Volume 1, Section 14.5.2).Also, certain detrimental bacteria are capable of breaking down glycerol, with a double dehydration reaction that produces acrolein ( Figure 2.5). Acrolein interacts with tannins to reinforce bitterness and is considered a type of microbial spoilage (acrolein spoilage or amertume ) (Section 8.3.2).
In view of its high concentration, it was thought that glycerol affected wine flavor, giving an impression of body and softness. In fact, doses much higher than those occurring naturally in wine are required to affect flavor to any significant extent.
2.3.2 C4 Polyols: 2,3‐Butanediol and Erythritol
The C4 molecule 2,3‐butanediol (Table 2.3) is a diol. It is a by‐product of alcoholic fermentation and is probably also formed by malolactic fermentation. This compound has little odor, and its taste is slightly sweet and bitter at the same time, but it does not have much organoleptic impact in wine. It is stable, and, above all, unaffected by bacteria.
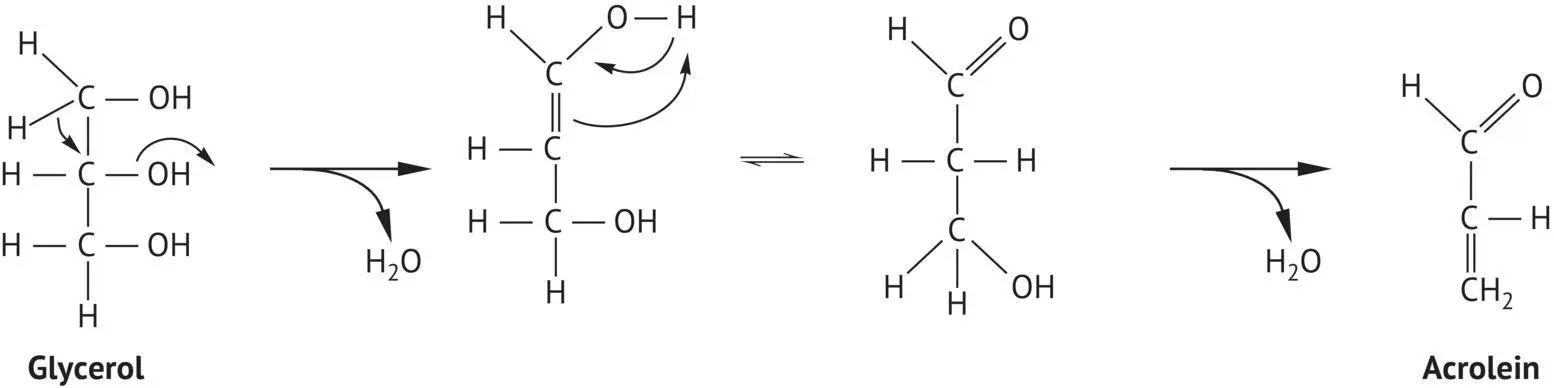
FIGURE 2.5 Mechanism for the formation of acrolein by double dehydration of glycerol.
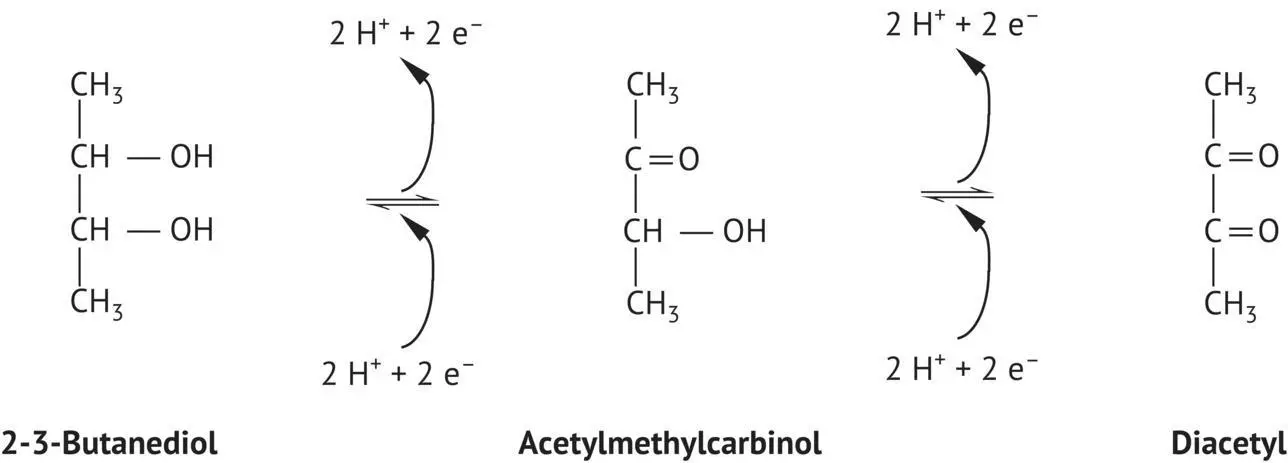
FIGURE 2.6 Oxidation–reduction equilibria of 2‐3‐butanediol.
The most significant role of 2,3‐butanediol is in maintaining an oxidation–reduction equilibrium with acetoin (or acetyl methyl carbinol) and diacetyl ( Figure 2.6). This compound (2,3‐butanediol) is formed following the reduction of acetoin, produced by the condensation of two acetaldehyde molecules.
Acetoin has a slight milky odor and is present at concentrations on the order of 10 mg/l. Diacetyl has a pleasant odor of butter, which may be perceptible at low concentrations (2 mg/l). The diacetyl concentration in wine is generally on the order of 0.3 mg/l.
These two volatile compounds are found in brandy. The concentration in brandy depends on that in the wine, and also on the distillation technique, making it possible to distinguish between Cognac, made by double distillation, and Armagnac, which is distilled only once.
Erythritol (Table 2.3) is also a C4 molecule, but it has four alcohol functions. Small quantities, 30–200 mg/l, are formed by yeast. It is not known to have any special properties.
2.3.3 C5 Polyol: Arabitol
Small quantities (25–350 mg/l) of arabitol are also known to be formed by yeast (Table 2.3). This compound has five alcohol functions and is directly derived from arabinose. Small quantities may also be produced by lactic acid bacteria and larger quantities by B. cinerea .
2.3.4 C6 Polyols: Mannitol, Sorbitol, and meso‐Inositol
These three compounds (Table 2.3) have six alcohol functions. The first two are linear, while the third is cyclic.
Mannitol is derived from the reduction of the aldehyde group on mannose. In wine, it is produced by the reduction of the ketone group on fructose by lactic acid bacteria. Mannitol is usually present in very small quantities. Higher concentrations are due to lactic acid bacteria or possibly B. cinerea . Abnormally high concentrations indicate severe lactic spoilage.
Sorbitol results from the reduction of the aldehyde group on glucose. This diastereoisomer of mannitol is totally absent from healthy grapes. Varying quantities are formed when B. cinerea develops. Alcoholic fermentation produces approximately 30 mg/l. Lactic acid bacteria do not form this compound. Large quantities of sorbitol indicate that wine has been mixed with fruit wines. Besides rowan berries ( Sorbus aucuparia , hence its name), apples, pears, and cherries also have a high sorbitol content.
Читать дальше















