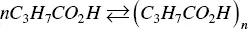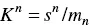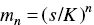1 ...6 7 8 10 11 12 ...49 Rising the surfactant concentration, the surface concentration increases as well until the full coverage of the interface by the molecules or ions. If the interface is completely covered, further increment of the surfactant concentration does not (almost) modify the surface tension. Furthermore, the additional surfactant molecules (or ions) have to remain in the bulk solution, and following Langmuir “hydrocarbon chains remain combined with each other, thus forming micelles” (or other aggregates).
The term micelle was commonly used by the first years of the twentieth century [40, 41] in relation to colloid solutions (frequently inorganic gels). In 1920 McBain and Salmon [42] (see also [43]) described a brief résumé of previous work, citing, for instance, Krafft's work. From the summary of this paper we extract the following sentences:
3. These colloidal electrolytes are salts in which one of the ions has been replaced by an ionic micelle.
5. This is exemplified by any one of the higher soaps simply on change of concentration. Thus, in concentrated solution there is little else present than colloid plus cation, whereas in dilute solution both undissociated and dissociated soap are crystalloids of simple molecular weight.
8. The ionic micelle in the case of soaps exhibits an equivalent conductivity quite equal to that of potassium ion. Its formula may correspond to but more probably it is , where P − is the anion of the fatty acid in question.
Therefore, the essential definition of the present concept of a micelle was established. IUPAC indicates that “Surfactants in solution are often association colloids, that is, they tend to form aggregates of colloidal dimensions, which exist in equilibrium with the molecules or ions from which they are formed. Such aggregates are termed micelles.”
In 1922, McBain and Jenkins [44] studied solutions of sodium oleate and potassium laurate by ultrafiltration, using this technique for separating the ionic micelle from the neutral colloid. For both surfactants they showed that the proportion (simple potassium laurate or sodium oleate)/(ionic micelle) increases fast at low concentrations and reached a plateau at high concentrations (see graphs of the paper). They also concluded that the diameter of the ionic micelle is only a few times the length of the molecule and “the particles of sodium oleate are about ten times larger than those of potassium laurate.”
By the end of the twenties and the beginning of thirties of the twentieth century, the research activity on micelle‐forming substances experienced an extraordinary blooming spring. The paper by Grindley and Bury [45] is a landmark on the subject, being particularly illustrative for the purposes of this review. They represented the formation of micelles by butyric acid in solution by the equation
(1.2) 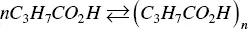
where n is “the number of simple molecules in a micelle” or aggregation number (which is a relatively large number) and write the equilibrium constant as
(1.3) 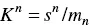
where s and m are the concentrations of butyric acid as monomers and as micelles, respectively. The previous equation can be written as
(1.4) 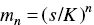
from which they deduced that if s/K is appreciably smaller than unity, the concentration of micelles will be negligible. Only when s approaches the value K does the concentration of micelles become appreciable, and “will rapidly increase as the total concentration increases.” From this analysis they conclude that “if any physical property of aqueous butyric acid solutions be plotted against the concentration, the slope of the curve will change abruptly near this point.” A few months later, Davies and Bury [46] named that concentration as the critical concentration for micelles .
Previous analysis constitutes the basis of all experimental techniques so far used for determining the critical concentration for micelles (from here cmc ). For instance, the association of monomers in micelles reduces the number of particles in the solution and, consequently, colligative properties (freezing point, vapor pressure…) also drastically change at this concentration. Other properties such as solubilization of solutes as dyes or the conductivity of the solution also change significantly. As an example, we shall mention the paper by Powney and Addison [47] who measured the surface tension of aqueous solutions of sodium dodecyl, tetradecyl, hexadecyl, and octadecyl sulfates and plotted the results in the form of vs log (concentration), as we do nowadays. The curves showed breaks at critical concentrations, which correspond to transitions from single ions to micelles, these single ions constituting the surface‐active species. Figure 1.3shows a typical plot for an unspecified surfactant. Powney and Addison noticed that the magnitude of the surface activity and the critical concentration for micelles were governed by chain length, temperature, and the valency of the added cation.
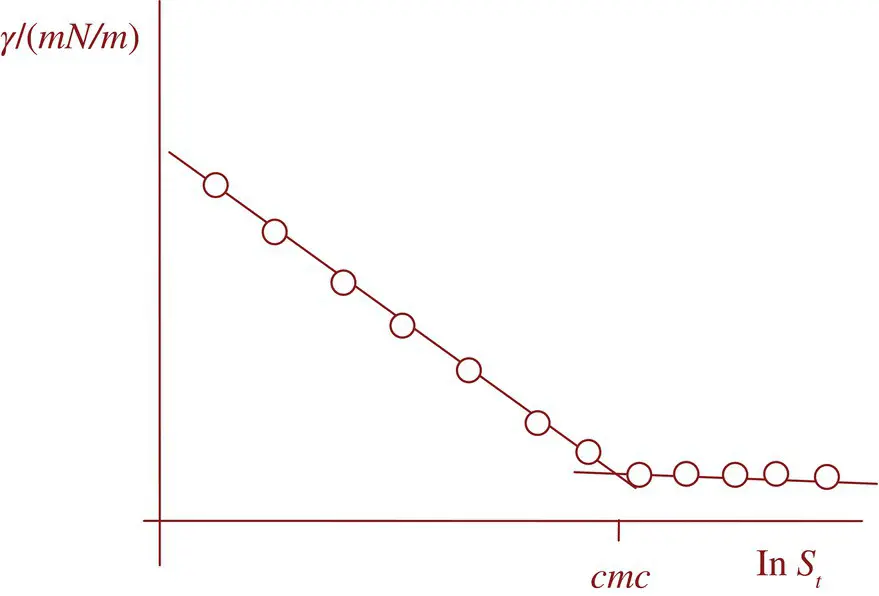
Figure 1.3 Typical surface tension vs ln (surfactant) plot showing the break point corresponding to cmc .
In 1895, Krafft and Wiglow [48] observed the formation of crystals at 60°, 45°, 31.5°, 11°, 35°, and 0° with hot aqueous solutions (1%) of stearate, palmitate, myristate, laurate, and elaidate sodium salts, respectively. Each of these temperatures is now known as the Krafft point ( T k). IUPAC defines it as the temperature (more precisely, narrow temperature range) above which the solubility of a surfactant rises sharply. At this temperature the solubility of the surfactant becomes equal to the cmc .
In 1955, Hutchinson et al. [49] published the paper “A new interpretation of the properties of colloidal electrolyte solutions” in which “the formation of micelles was treated as a phase separation rather than as an association governed by the law of mass action.” Seven years later, Shinoda and Hutchinson [50] used this model to interpret the Krafft point, associated to the micellization process. These authors proposed micellization as a “similar phase separation, with the important distinction that micellization does not lead to an effectively infinite aggregation number, such as corresponds to true phase separation.” If correct, the model requires that the activity of micelle‐forming compounds should be practically constant above the cmc . Among others, the authors invoke Nilsson results [51] with radiotracers as evidence for their proposition. In a frequently reproduced graph, Shinoda and Hutchinson [50] plotted the concentration vs temperature for sodium decyl sulfonate near the Krafft point. The plot resembles the phase diagram of water near its triple point. If micelles are considered as a phase, by the phase rule, the system should become invariant at constant temperature and pressure [49, 50]. In other words, “the equilibrium hydrated solid monomers micelles is univariant, so that at a given pressure the point is fixed.” As temperature increases, the solubility also increases until T kwhere the cmc is reached. Above this temperature, the surfactant is dissolved in the form of micelles.
Читать дальше