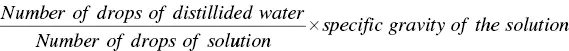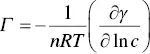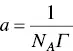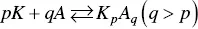1 ...7 8 9 11 12 13 ...49 Let us go back to 1915. In this year, Allen [52] published a paper in which he showed the use of the surface tension measurement for the determination of bile salts in urine. In the introduction of his paper, Allen refers to Hay’s method of testing the presence of bile salts in the urine. That method consists in “shaking flowers of sulphur upon the surface of the urine… When the surface tension of the urine is lowered [by bile salts] the powdered sulphur sinks to the bottom, and the lower the surface tension the more rapidly this takes place. [But] The method is very unsatisfactory… and if possible, a quantitative method, would be very desirable.” Thus Allen proposed a very accurate measurement of the surface tension of a solution by the stalagmometric method “to determine the feasibility of estimating the amount of bile salts present in pathological urines from measurements of the surface tension taken with a portable Traube stalagmometer.” He computed the surface tension value of a solution in per cent of that of distilled water according to the formula
(1.5) 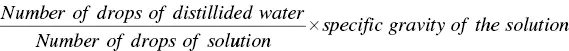
The method relies on the fact that bile salts possess the property of lowering the surface tension of a solution very markedly, even when present in small concentrations. Table I of his paper shows some results for sodium glycocholate (NaGC) in distilled water. In a reanalysis of these data by plotting the surface tension vs ln(concentration), it is possible to determine a value of 0.0117 M for the cmc of NaGC, a value in perfect agreement with recent measurements. From the Reis et al. [53] compilation, an average value of (1.04 ± 0.29) × 10 −2M may be estimated for the cmc of this bile salt.
The “excess of solute [surfactant] in the surface” indicated by Milner has traditionally been analyzed through the Gibbs equation [54]
(1.6) 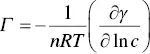
where Γ is the surface excess, ( ∂γ /∂ln c ) is the slope of the dependence of γ with the logarithm of the concentration of the surfactant (frequently being linear), R is the ideal gas constant, T the temperature, and n a factor that depends on the nature of the surfactant. The equation allows the determination of the area occupied per molecule at the interface, which is the inverse of the surface excess, i.e.
(1.7) 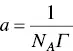
where N Ais Avogadro's number.
Recently Menger et al. [55] have questioned the validity of the Gibbs equation on the basis that in the region of concentration where the equation is applied the adsorption at the interface does not generally reach saturation. This criticism has been supported by measurements from a radioactive surfactant [56], results that suggest that the γ ‐ln c linearity is not indicative of surface saturation, a hypothesis required for the deduction of the Gibbs equation. Neutron reflection measurements also support the fact that there are serious limitations in applying the Gibbs equation accurately to surface tension data [57, 58].
In the late nineteen thirties, other important papers were published. Wright and co‐workers [59–61] measured the conductivity, density, viscosity, and solubility of several sodium alkyl (decyl, dodecyl, and hexadecyl) sulfonates at several temperatures. In all cases breaks at the curves or linear dependences of the property with the sulfonate concentration were observed. They also reported that the addition of sodium chloride to solutions of sodium dodecyl sulfonate lowered the cmc and that the lowering becomes less marked with a rise in temperature. Hartley [62] demonstrated that paraffin chain salts behave as strong electrolytes at low concentrations. For cetane sulfonic acid, a value of about 0.008 N in water at 60 °C was given for cmc and that it increased by about 2% per degree. This is an important question since the formation (or not) of premicellar aggregates is still under debate.
By the end of this decade Hartley [63] reviewed (36 references) the subject, the title of the paper being Ion aggregation in solutions of salts with long paraffin chains . In the abstracts we can read about the structure of micelles which are “aggregates of paraffin‐chain ions with some adsorbed opposite ions,” micelles are spherical with a radius equal to the length of a completely stretched paraffin‐chain and have a liquid interior and the strong dependence of cmc with the length of the hydrocarbon chain and nature of the ionized terminal groups and opposite ions (counterions), and with temperature (in less extension). He also affirmed that “the spherical micelle is more stable than ion pairs.”
Thus, by this time, the essential parameters that define a micelle were introduced or established: change of properties at the cmc , variables that influence the cmc , shape and size, internal and peripheral structures, and the essential thermodynamics (mass action law).
In the period 1946–1947, immediately after the Second World War, the activity on surfactant research experiences an important enhancement.
Corrin and Harkins [64] proposed the equation log( cmc ) = − A × log( counterion ±) − B to relate the dependence of the cmc with the concentration of added salts (the sign at the superscript of the counterion is opposite to that of the surfactant ion). Table 1.1resumes the values for the constants A and B for several surfactants. They also noticed that urea has a negligible effect in lowering the cmc .
Table 1.1 Parameters A and B of the Corrin–Harkins equation. The number of figures on the values of A and B has been reduced.
Source: Corrin and Harkins [64], p. 683 .
| Surfactant |
A |
B |
| Potassium laurate |
0.570 |
2.62 |
| Sodium dodecyl sulfate |
0.458 |
3.25 |
| Dodecyl ammonium chloride |
0.562 |
2.86 |
| Decyltrimethylammonium bromide |
0.343 |
1.58 |
Three years later, Lange [65] applied the mass action law to ionic micelles and wrote the equilibrium of formation of the micelle as
(1.8) 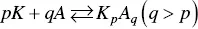
where K is the counterion, A the surfactant ion, and p and q the stoichiometric coefficients. Although Lange considered the activity coefficients of the different species, for simplicity we will ignore them and write the equilibrium constant as
(1.9) 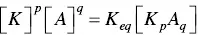
Writing [ A ] = c kand [ K ] = c k+ N , where N is the equivalent concentration of added salt, it is finally found that
(1.10) 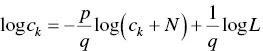
Читать дальше