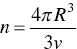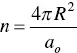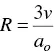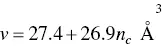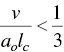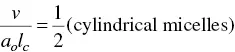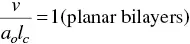After several assumptions (micellar distribution is Gaussian‐like, reactions between aggregates do not have to be considered, and the change in the number of the micelles is much slower than the interchange of the monomer) Eq. (1.29)is deduced [140]:
(1.29) 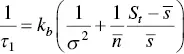
where τ 1is the relaxation time (associated to k b), σ 2is the variance of the micellar distribution, 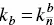 is the backward rate constant at micelle sizes around the mean micelle size
is the backward rate constant at micelle sizes around the mean micelle size 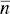 , and
, and 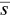 is the average monomer concentration. In addition,
is the average monomer concentration. In addition, 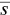 is identified with the concentration at cmc , s cmc, at concentrations above the cmc . When
is identified with the concentration at cmc , s cmc, at concentrations above the cmc . When 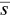 , k b, the aggregation number and the standard deviation (see above) do not appreciably change with the concentration, and the previous equation suggests a linear relationship between
, k b, the aggregation number and the standard deviation (see above) do not appreciably change with the concentration, and the previous equation suggests a linear relationship between 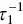 and the concentration. This fact has been verified, for instance, for alkyl sulfates [128].
and the concentration. This fact has been verified, for instance, for alkyl sulfates [128].
The backward kinetic constant depends on the length of the alkyl chain of the surfactant. For instance, values in the interval 10–0.8 × 10 9/s have been measured by Kaatze [136] for ammonium chloride surfactants CH 3C x−1H 2(x−1)NH 3 +Cl −( x = 5–8).
1.4 Packing Properties of Amphiphiles
In 1964 Reiss‐Husson and Luzzati [141] used small‐angle X‐ray scattering methods to study micellar solutions of several amphiphiles in water, without added electrolytes. The amphiphiles were sodium salts of lauryl sulfate (SLS), laurate (NaC 12), myristate, palmitate, stearate and oleate, and cetyltrimethylammonium chloride (CTACl) and bromide. The studies were performed at different concentrations and temperatures. SLS (67, 25 °C), NaC 12(25, 70 °C), and CTACl (84, 27 °C) form spherical micelles with the aggregation numbers given in parenthesis. However, at high surfactant concentrations the spheres become rods and the concentration at which the sphere–rod transition takes place was also determined.
The sphere–rod transition was observed by Hayashi and Ikeda [142] for SLS when the concentration of NaCl is increased. The transition is accompanied by a large increment of the aggregation number. For instance, at NaCl 0.01 M the apparent aggregation number is 70 while at NaCl 0.80 M the value is 1630 and the length of the rod 597 Å. A simple version of this experiment was provided by Coello et al. [143].
The monomer packing of an alkyl surfactant in a given geometrical shape, for instance, a sphere, will depend on parameters such as the micellar radius, R , chain monomer volume, v , maximum length that the chain can assume (critical chain length, l c), and the interfacial area per monomer, a o. For spherical micelles, simple geometry gives [125],
(1.30) 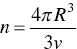
(from the volume) and
(1.31) 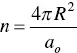
(from the surface). It follows that
(1.32) 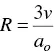
According to Tanford [144], for an alkyl chain with n ccarbon atoms the maximum chain length is given by
(1.33) 
and the volume of the alkyl chain as
(1.34) 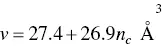
Only with the condition ( l ccritical length)
(1.35) 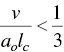
will the amphiphiles be able to fill the whole volume of the spherical micelle without leaving an empty space in the micelle core. For two surfactants having identical a ovalues, previous equations predict that both the radius ( R 2= ( v 2/ v 1) 2 R 1) and the aggregation number ( n 2= ( v 2/ v 1) 2 n 1) will increase with the alkyl chain length. Figure 1.5shows the plot of ln n vs ln v for sodium alkyl sulphates at 25 °C [145] and sodium alkyl sulfonates at 23–60 °C [76], the values of the slopes being 2.06 ± 0.05 and 2.20 ± 0.11, respectively, in good agreement with the theoretical expected one equal to 2. For the original measurements of Debye the value of the slope is 3.53. The value of a odepends not only on the structure of the hydrophilic head group but also on the environmental properties (presence of inert salts, pH, additives, temperature).
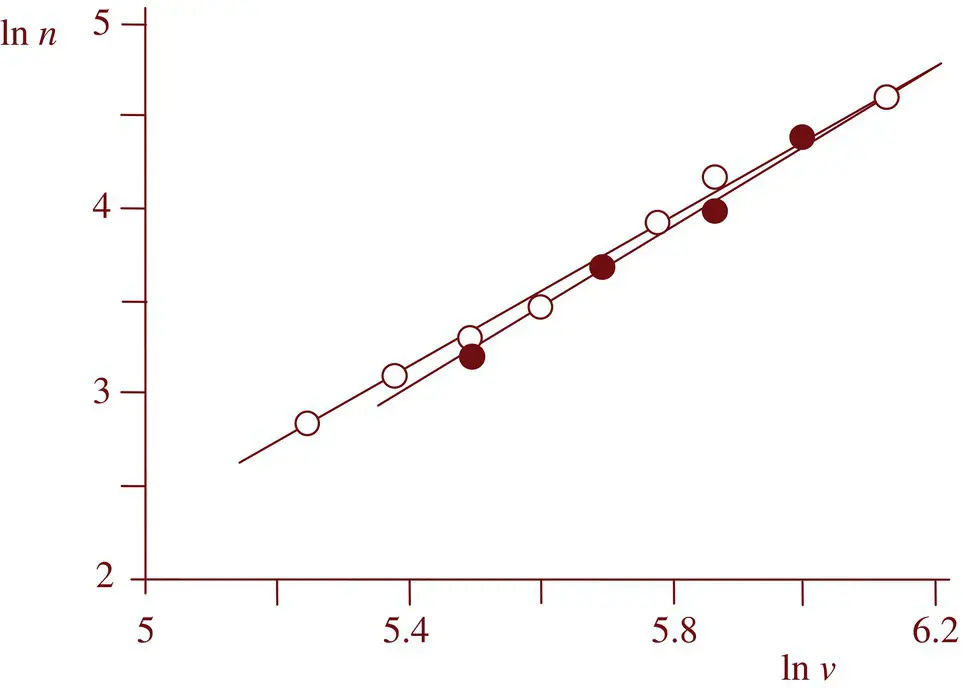
Figure 1.5 Plot of logarithm (aggregation number) vs logarithm (alkyl chain volume) of sodium alkyl sulfates (o) and sodium alkyl sulfonates (•).
Source: Original data from Aniansson et al. [145] and Tartar and Lelong [109], respectively.
The ratio ( v/a o l c) of Eq. (1.35)is named as packing parameter P .
Surfactants can self‐aggregate in other well‐defined structures, different from spherical, ellipsoidal or rod‐like micelles. Flat lamellar or disk‐like structures are also common. Jung et al. [146] have studied the origins of stability of spontaneous vesicles. The cryo‐TEM images provided by these authors, unequivocally show the formation of vesicles with a well‐defined limiting membrane [147]. Similarly, Terech and Talmon [148] have demonstrated the formation of long single‐walled tubes, and mechanisms for the formation of these tubes have been proposed for other bile acid derivatives [149, 150].
The determining factor of the surfactant molecules to self‐organize in one or another structure is the packing parameter P . Israelachvili et al. [124] have indicated that the critical conditions for cylindrical micelles and planar bilayers are
(1.36) 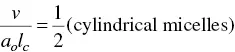
(1.37) 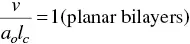
Читать дальше

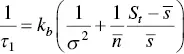
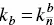 is the backward rate constant at micelle sizes around the mean micelle size
is the backward rate constant at micelle sizes around the mean micelle size 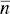 , and
, and 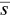 is the average monomer concentration. In addition,
is the average monomer concentration. In addition, 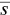 is identified with the concentration at cmc , s cmc, at concentrations above the cmc . When
is identified with the concentration at cmc , s cmc, at concentrations above the cmc . When 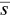 , k b, the aggregation number and the standard deviation (see above) do not appreciably change with the concentration, and the previous equation suggests a linear relationship between
, k b, the aggregation number and the standard deviation (see above) do not appreciably change with the concentration, and the previous equation suggests a linear relationship between 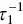 and the concentration. This fact has been verified, for instance, for alkyl sulfates [128].
and the concentration. This fact has been verified, for instance, for alkyl sulfates [128].