The structure of gemini surfactants [151, 152] provides different ways of changing their physicochemical properties. The ionic groups may be positive, negative, or zwitterionic, the spacer may be rigid or flexible allowing for different separation lengths between the hydrophilic groups, and the hydrophobic chains may be identical or not. In this last case, the gemini is clearly asymmetric. Thus, different values can be obtained for the packing parameter. A particularly instructive example is the family of zwitterionic geminis synthesized by Peresypkin and Menger [153] in which the negative group is a phosphodiester and the other is a cationic quaternary ammonium salt ( Scheme 1.2).
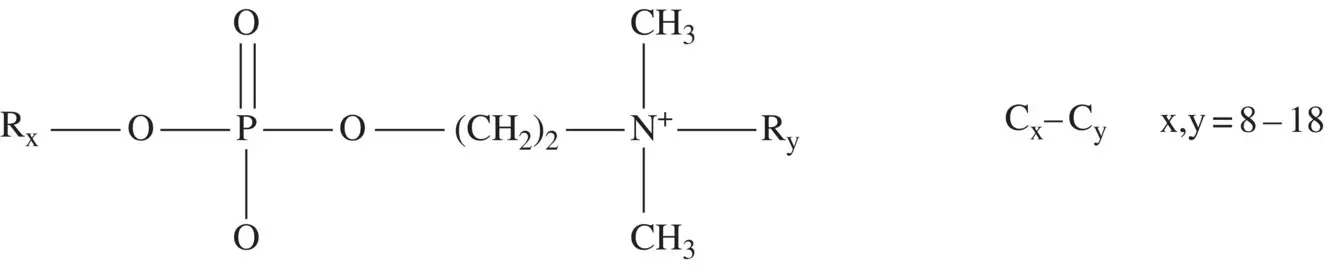
Scheme 1.2 Structure of synthetized gemini surfactants.
Source: Peresypkin and Menger [153].
Dynamic light scattering experiments suggest that only the C8–C8 derivative originates micelles exclusively, while others (for example, C8–C12, C12–C8, C14–C8, C10–C14, and C10–C16) self‐assemble in structures such as tubules or vesicles of different sizes, forming also coacervate droplets (C8–C10).
Although phospholipids or bile acids are of a biological origin, the term “biosurfactant” concerns those amphiphilic derivatives that are produced by microorganisms. In general, they have excellent surface‐active properties.
Different classifications have been purposed for biosurfactants [154–156]. For instance, Otzen indicates that, based on their overall structure, biosurfactants fall into four classes: glycolipids, lipopeptides, saponins, and all the rest. Glycolipids can be subdivided into rhamnolipids, sophorolipids, trehalolipids …, in which the head group are different saccharides (rhamnose, sophorose, trehalose, …). Similarly, lipopeptides can be divided into several families as surfactin, iturin or fengycin [157, 158], some of them having a peptide‐cycle structure. Gemini type biosurfactants are also common [159]. The hydrophobic part is commonly one or more unsaturated or saturated hydrocarbon chains. The saponin Aescin or glycyrrhic acid are examples of biosurfactant complex structures [159, 160].
The number of biosurfactants can enormously be enlarged by chemical modifications, which can be carried out in laboratories. Sugar surfactants are well‐known examples in which glucose, galactose, xylose, sucrose, or lactose are common hydrophilic heads [161]. They can easily be obtained chemically [162] or enzymatically [163]. Baccile et al. [26] have obtained and characterized the self‐assembly properties of a broad family of amino derivatives of sophorolipid biosurfactants, including asymmetric (sophorose–ammonium and sophorose–amine oxide) and symmetric (sophorose–sophorose bearing three and four hydrophilic centers) bolaamphiphiles.
Previous naturally found biosurfactants and similar derivatives will all together show a broad range of physicochemical properties. Let us analyze some significant examples.
The surface tension vs concentration curves of biosurfactants are similar to those of classical surfactants. The break points in these plots, corresponding to cmc values, are normally well defined, and the surface tension values at cmc , γ cmcare frequently found to be <30 mN/m. However, differences in reported cmc values are not uncommon, probably due to the presence of impurities or the use of mixtures instead of pure samples. For instance, Saini et al. [164] reported a value of 54 mg/l for the cmc of viscosin ( Figure 1.6), a cyclic oligopeptide lipid which contains nine amino acids: two (leu‐glu) are linked to the fatty acid tail, and the remaining seven [val‐leu‐ser‐leu‐ser‐ile‐allo(thr)] form a cycle. However, other values have also been reported for cmc , as Saini et al. have noticed, with values ranging from 4 to 9 mg/l [165] to 150 mg/l [166].
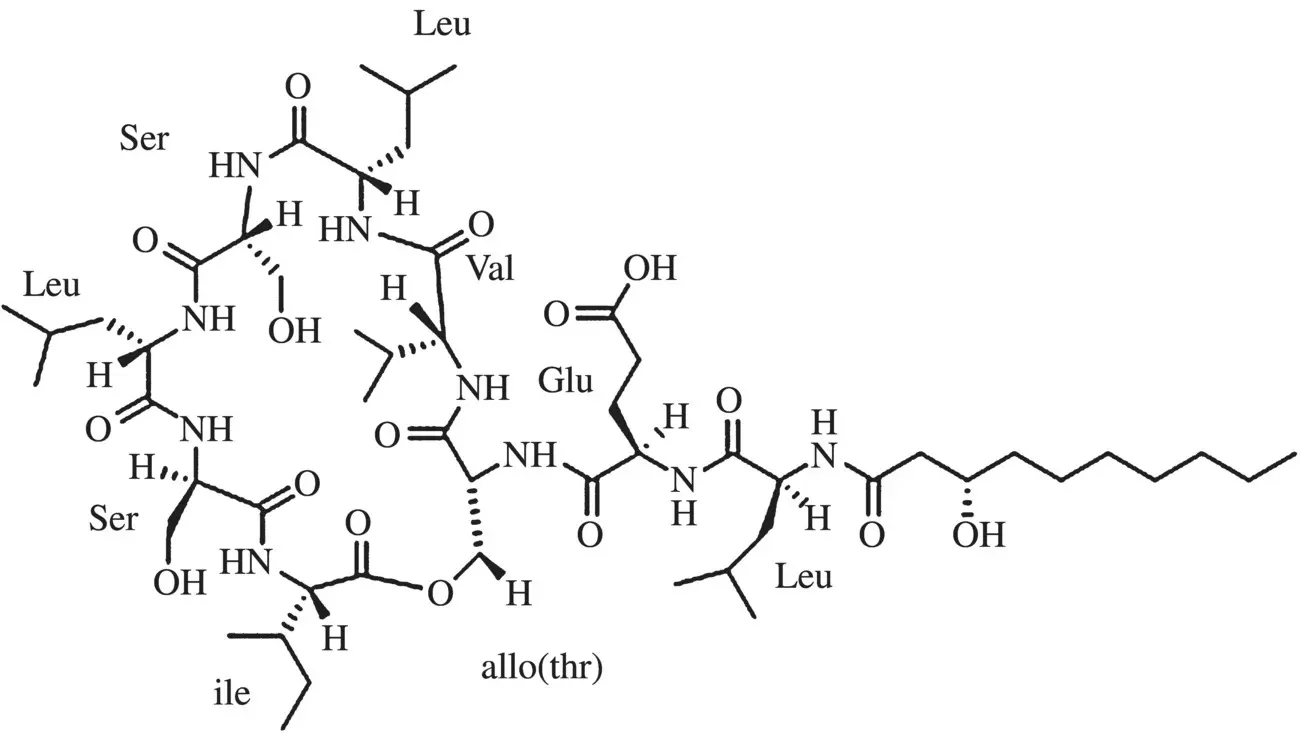
Figure 1.6 Structure of viscosin.
Source: Saini et al. [164]. Reproduced by permission of the American Chemical Society.
General rules observed in classical surfactants are also observed for biosurfactants. Figure 1.5shows a linear relationship between the aggregation number and the alkyl chain volume in classical surfactants. This is just an example of linear relationships for different properties in a series of homologous surfactants. For instance, Garofalakis et al. [161] have observed a reduction in the critical aggregation concentration ( cac ) of the surfactants when increasing the carbon chain length for a series of monoesters of xylose, galactose, sucrose, and lactose with different hydrophobic chain lengths (C12–C16). These authors also observed that the more hydrophilic head groups, the higher is cac , though this trend was moderated by the alkyl chain length. Some observed differences between maltose and glucose derivatives have been ascribed to a higher degree of hydration of maltose compared to that of the glucose head group as molar heat capacities for these two sugars suggest [167]. Another example corresponds to the standard free energy change. For n c‐alkyl‐D‐maltosides ( n c, number of carbon atoms of the alkyl chain, = 8, 10, 12, and 14), Varga et al. [168] have shown that the dependence of the standard free energy change (and from here the cmc as well) with the length of the alkyl chain for these sugar surfactants is parallel to the one for alkyltrimethylammonium bromides and sodium alkylsulfates.
Similarly, Ribeiro et al. [169] have obtained diacetylated lactonic sophorolipids with different hydrophobic chains (C18:0, C18:1, C18:2, and C18:3). The cmc values (in mg/l) increase linearly with the number of double bonds from 29.2 (C18:0) to 39.1 (C18:3), the slope being 3.35 ( r 2= 0.979). The surface tension at cmc ( γ cmc) also increases with that number (from 35.7 to 38.8 mN/m).
Sugar alkyl surfactants also display additivities with the number of carbon atoms of the alkyl chain. For instance, Angarten and Loh [170] studied two series of surfactants C nc G x using ITC, where x = 1,2 is the number of glucoside units (G) of the hydrophilic head and n c= 7–12 (the limits depending on x ). Both families exhibit a linear Δ C pvs n cplot, the slope representing the contribution of each methylene group to the heat capacity for micellization. The obtained values for the slope are 63 ± 2 J/(K mol) and 59 ± 2 J/(K mol) for x = 1 and x = 2, respectively. Therefore, within experimental error, there are not significant differences between them, suggesting that the contributions of the mono or diglucoside head groups are essentially the same. Let us remember that a value of 33 J/(K mol) was found for the contribution of each hydrogen atom of each methylene group for the removal of alkanes from water to air [171]. This also has been related to the number of water molecules in the first solvation shell that contribute to the thermodynamics of hydrophobic solvation [102]. Other ITC measurements for alkyl sugar surfactants have been carried out by Blume et al. [92, 172]. Similar studies [173] were carried out for monorhamnose and dirhamnose rhamnolipids (R1, R2) (and their mixtures) ( Figure 1.7). The cmc values are shown at Table 1.2.
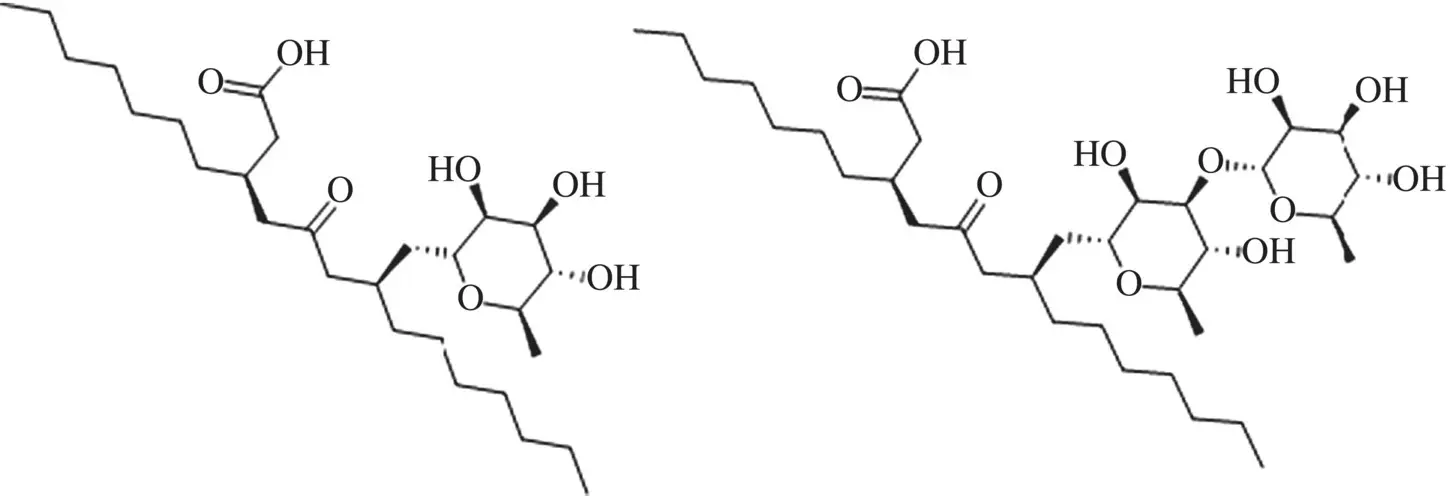
Figure 1.7 Structure of biosurfactants monorhamnose and dirhamnose rhamnolipids (R1 ‐left‐ and R2 right).
Читать дальше















