Starting from a model published by Burchfield and Woolley [120], Coello et al. [121, 122] combined freezing point and sodium ion activities measurements to obtain both the aggregation number and the fraction of bound counterions of aqueous bile salt solutions. The mass action model for an ionic micelle and the fraction of bound counterions to the micelles were considered. The theory of the freezing point depression is very well known [54] and does not need further attention.
While in a monodisperse system (as we have analyzed above) the aggregation number is a single‐value variable [123], in a polydisperse self‐aggregation system the aggregation number can assume all possible values from 2 to ∞. For these systems only the average aggregation number of the aggregates is obtained. However, different experimental techniques lead to aggregation numbers that are not the same. For instance, the average aggregation number measured from colligative properties is the number average aggregation number, 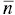 , given by
, given by
(1.22) 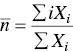
where X iis the concentration of the i th species. Similarly, the average aggregation numbers obtained from static light scattering and viscosity techniques are the weight average and the z ‐average aggregation numbers, 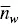 and
and 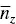 , respectively, given by
, respectively, given by
(1.23) 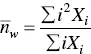
(1.24) 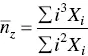
For analyzing the experimental results from the experimental techniques, the hypothesis of a constant average aggregation number with concentration is frequently used. Let us examine this hypothesis by following the landmark paper by Israelachvili et al. [124]. In this presentation we are following the notation of this original paper.
Each aggregate in the solution is characterized by its own free energy. They also accepted that the dilute solution theory holds. Equilibrium thermodynamics requires that in a system of molecules that form aggregated structures in solution, the chemical potential of all identical molecules in different aggregates is the same [125]. For an aggregate containing i monomer molecules, this statement is expressed as
(1.25) 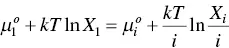
where  is the standard part of the chemical potential (the mean interaction free energy per molecule) in aggregates of aggregation number i and X iis the concentration of the i th aggregate. The subscript “1” corresponds to monomers in solution. That is to say, the left‐side term corresponds to the chemical potential of the free monomer and the right‐side term is the chemical potential per monomer incorporated in a micelle.
is the standard part of the chemical potential (the mean interaction free energy per molecule) in aggregates of aggregation number i and X iis the concentration of the i th aggregate. The subscript “1” corresponds to monomers in solution. That is to say, the left‐side term corresponds to the chemical potential of the free monomer and the right‐side term is the chemical potential per monomer incorporated in a micelle.
From the previous equation and the material balance in a polydispersed micellar solution, it follows that the number average aggregation number is [124]
(1.26) 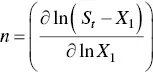
which relates the rate of change of micelle concentration with monomer concentration to the mean micelle aggregation number. It should be noticed that this equation reduces to Eq. (1.19)for a monodisperse system. In such a case, the various average aggregation numbers are identical to n [123].
The standard deviation is given by
(1.27) 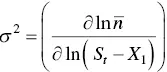
which relates it to the rate of change of the mean aggregation number with respect to micelle concentration and means that if the standard deviation is close to zero, the aggregation number must almost be independent of the total surfactant concentration.
Well above cmc , Eq. (1.27)may be approximated by [125]
(1.28) 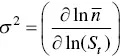
which shows that if the system is highly polydisperse the average aggregation number will be very sensitive to the total surfactant concentration. Therefore, a rapid change of concentration is evidence of a large distribution of polydispersity in micelle size. Nagarajan [123] remarks that “this is a general thermodynamic result applicable to any self‐assembling system” and that “interpreting any experimental data, one must ensure that this equation is not violated.” Similar comments have been provided by Rusanov [126].
A well‐known approach to study a fast process is to rapidly perturb a system in equilibrium and measure the relaxation time required by the system to adjust to the new equilibrium. Typical techniques are temperature jump, pressure jump, and ultrasonics [127]. The time scale depends on the relaxation technique [128]. The mathematical analysis to study simple A↔B and A+B↔C systems by temperature jump has been didactically published by Finholt [129]. Kresheck et al. [130] applied the temperature‐jump (jump 5.2 °C) technique to study the dissociation of the dodecylpyridinium iodide micelle. Pressure‐jump studies have also been carried out by several authors [131–133].
In the ultrasonic methods (Ultrasonic Absorption Spectrometry) the perturbation of equilibrium is due to the periodic variation of pressure and temperature due to the passage of the sound wave through the system. Relaxation of the equilibrium gives rise to changes in the quantity α / f 2(where α is the sound absorption coefficient at frequency f ) and from its dependence with frequency the relaxation time is obtained [128].
Platz [134] has presented a simplified analysis of the Aniansson and Wall [135] isodesmic model for analyzing the kinetics of micelle association and dissociation in surfactant solutions. The theory is nowadays commonly known as the Teubner–Kahlweit–Aniansson–Wall theory [136, 137], the key equation being Eq. (1.14), characterized by forward ( 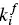 ) and back
) and back 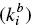 kinetic constants, which correspond to the exchange of monomers between micelles. The ratio between the kinetic constants is directly the equilibrium constant. Further developments of the theory are due to Lang et al. [133] and Telgmann and Kaatze [138, 139]. In relaxation experiments two characteristic times are observed. The so‐called “fast process” corresponds to the kinetic analysis of Aniansson and Wall and the “slow process” is assumed to be a change of the total number of micelles.
kinetic constants, which correspond to the exchange of monomers between micelles. The ratio between the kinetic constants is directly the equilibrium constant. Further developments of the theory are due to Lang et al. [133] and Telgmann and Kaatze [138, 139]. In relaxation experiments two characteristic times are observed. The so‐called “fast process” corresponds to the kinetic analysis of Aniansson and Wall and the “slow process” is assumed to be a change of the total number of micelles.
Читать дальше

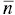 , given by
, given by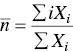
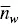 and
and 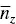 , respectively, given by
, respectively, given by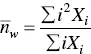
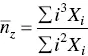
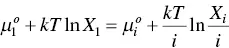
 is the standard part of the chemical potential (the mean interaction free energy per molecule) in aggregates of aggregation number i and X iis the concentration of the i th aggregate. The subscript “1” corresponds to monomers in solution. That is to say, the left‐side term corresponds to the chemical potential of the free monomer and the right‐side term is the chemical potential per monomer incorporated in a micelle.
is the standard part of the chemical potential (the mean interaction free energy per molecule) in aggregates of aggregation number i and X iis the concentration of the i th aggregate. The subscript “1” corresponds to monomers in solution. That is to say, the left‐side term corresponds to the chemical potential of the free monomer and the right‐side term is the chemical potential per monomer incorporated in a micelle.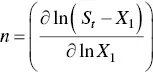
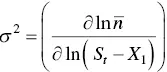
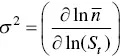
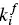 ) and back
) and back 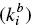 kinetic constants, which correspond to the exchange of monomers between micelles. The ratio between the kinetic constants is directly the equilibrium constant. Further developments of the theory are due to Lang et al. [133] and Telgmann and Kaatze [138, 139]. In relaxation experiments two characteristic times are observed. The so‐called “fast process” corresponds to the kinetic analysis of Aniansson and Wall and the “slow process” is assumed to be a change of the total number of micelles.
kinetic constants, which correspond to the exchange of monomers between micelles. The ratio between the kinetic constants is directly the equilibrium constant. Further developments of the theory are due to Lang et al. [133] and Telgmann and Kaatze [138, 139]. In relaxation experiments two characteristic times are observed. The so‐called “fast process” corresponds to the kinetic analysis of Aniansson and Wall and the “slow process” is assumed to be a change of the total number of micelles.










