2.2.2 The Process and Methods
Figure 2.1provides key differences in the process of conducting IPD meta‐analysis projects compared to conventional reviews based on aggregate data. 7,43Best practice is to publish and adhere to a protocol, regardless of whether aggregate data or IPD are being used, although protocols for IPD projects will usually be more detailed ( Section 4.2.2). Methods for identifying trials are very similar in the two approaches, but in an IPD project searches may be conducted prior to or in tandem with protocol development, in order to generate a preliminary list of trials ( Section 4.2.3), and to identify the associated investigators from whom IPD will be sought ( Section 3.2).
Prior to data collection, an IPD project may require ethical approval ( Section 3.10) and development of formal data‐sharing agreements ( Section 3.11), as well as the preparation of a detailed data dictionary ( Section 4.2.7). These are rarely required for an aggregate data review. Furthermore, the subsequent data collection, checking and analytical aspects of an IPD project are much more exacting than those for aggregate data reviews. They may include data entry, data re‐coding and harmonisation, together with checking, querying and subsequent validation of IPD with original trial investigators ( Chapter 4), 7,43,44as well as advanced statistical methods for meta‐analysis (Part 2).
Unlike aggregate data reviews, IPD projects usually involve and benefit from establishing partnerships with trial investigators who, in addition to providing their IPD, play an active role throughout the process, from identifying relevant trials through to helping interpret and disseminate IPD meta‐analysis results. This may include establishing a collaborative group that authors the main project publication, with all those involved being listed as co‐authors, and holding a meeting of this Group where preliminary results are presented and discussed ( Section 3.8). 7,43Recently, a range of clinical study data repositories and platforms have been established, offering another source of IPD from existing trials, but there are both advantages and disadvantages of obtaining for IPD meta‐analysis projects in this way ( Sections 3.2.2and 4.4.5). 45
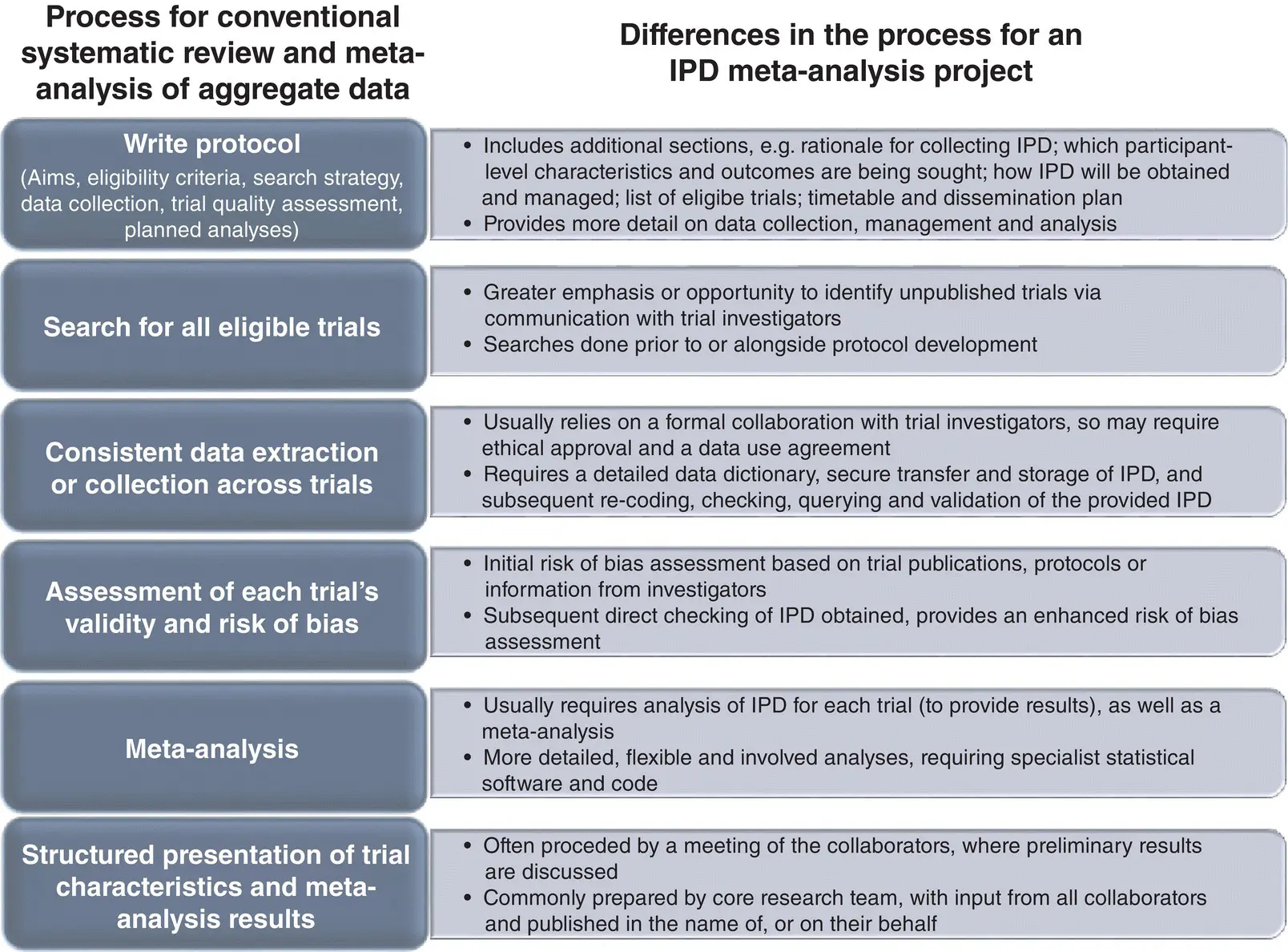
Figure 2.1Key differences between the process for a IPD meta‐analysis project and a conventional systematic review and meta‐analysis of aggregate data.
Source: Jayne Tierney.
Given these differences, IPD meta‐analysis projects require a greater range of skills ( Section 3.5), generally take longer ( Section 3.7), and need more resources ( Section 3.8) than traditional systematic reviews and meta‐analyses based on aggregate data.
2.3 What Are the Potential Advantages of an IPD Meta‐Analysis Project?
Provided it is conducted appropriately, an IPD meta‐analysis project offers many advantages over the conventional aggregate data approach ( Table 2.1). 7,9,43,44A key benefit is the potential to improve the quantity and quality of data, because there is no need to be limited by what has been published. For example, IPD from unpublished trials can be included ( Section 4.2.3), as can any outcomes that were not reported for published trials, or even participants who were inappropriately excluded from the original trial analyses. 7,9,43As well as helping to circumvent potential reporting biases, 46this can increase the quantity of information available for analysis and, therefore, boost the statistical power to detect genuine effects. 47In addition, there is greater ability to standardise outcome and covariate definitions across trials ( Section 4.5), which not only facilitates the conduct of meta‐analysis, but also aids the interpretation of findings. Detailed data checking helps to ensure the completeness, validity and internal consistency of data items for each trial, further enhancing data quality ( Section 4.5.4), 7,9,43,44as well as providing independent scrutiny of the trial data.
Table 2.1Key potential advantages of an IPD meta‐analysis project compared with a conventional systematic review and meta‐analysis of aggregate data focusing on the synthesis of randomised trials to evaluate treatment effects, adapting those shown by Tierney et al. 9
Source: Adapted from Tierney et al., 9with permission, © 2015 Tierney et al. (CC BY 4.0).
| Aspect of systematic review or meta‐analysis |
Advantages of an IPD meta‐analysis project |
| Trial identification and inclusion |
Ask collaborative group (trial investigators and other experts in the clinical field) to help identify eligible trials (particularly those that are unpublished or ongoing) * Clarify a trial’s eligibility with the trial’s investigators * |
| Data completeness and uniformity |
Include data from trials that are unpublished or not reported in full * Include unreported data (e.g. unpublished subgroups, outcomes and time‐points), more complete information on outcomes, and data on participants excluded from original trial analyses * Check each trial’s IPD for completeness, validity and consistency, and resolve any queries with trial investigatorsDerive new or standardised outcome definitions across trials or translate different definitions to a common scaleDerive new or standardised classifications of participant‐level characteristics, or translate different definitions to a common scaleUpdate follow‐up of time‐to‐event or other time‐related outcomes beyond those reported * |
| Risk of bias assessment |
Clarify trial design, conduct and analysis methods with trial investigators * Resolve unclear risk of bias assessments (i.e. based on trial reports) through direct contact with investigators * Examine trial IPD directly for evidence of potential bias in trial design and conduct, and resolve any queries with trial investigatorsObtain extra data where necessary to alleviate or mitigate against potential biases * |
| Analyses |
Apply a consistent method of analysis for each trial (independent of original trial analyses)Analyse all important outcomes irrespective of whether published * Explore validity of analytical assumptions e.g. normality of residuals in a linear regression analysisDerive outcomes and measures of effect directly from IPD (independent of trial reporting), potentially at multiple time‐points of interestUse a consistent unit of analysis for each trial (e.g. consistently analyse preterm birth events per mother rather a mix of per mother and per baby in trials that include twin pregnancies)Account for complexities in each trial in the analysis, such as cluster randomised trials or multi‐centre trialsAnalyse continuous outcomes on their continuous scale and adjust for baseline valueAdjust for a pre‐defined set of prognostic factorsApply consistent definitions for categorised data (e.g. stage of cancer)Conduct more detailed and appropriate analysis of time‐to‐event outcomes (e.g. handling of censored observations, generating Kaplan Meier curves, examination of non‐proportional hazards)Achieve greater power for assessing interactions between effects of interventions and participant‐level characteristicsModel associations at the participant level, including potential non‐linear relationshipsUse appropriate but non‐standard models (e.g. that account for repeated measurements or correlation between multiple outcomes) or measures of effectExplain potential heterogeneity and inconsistency in network meta‐analysisAddress additional important questions over and above efficacy, or not considered by original trials e.g. to explore the natural history of disease, prognostic factors or surrogate outcomes |
| Interpretation |
Discuss implications for clinical practice and research with a multi‐disciplinary group of collaborators including trial investigators who supplied data, and patient research partners * |
| Dissemination |
Achieve more widespread dissemination though collaborative group networks and patient groups |
* These advantages accrue from direct contact with trial investigators (rather than the IPD per se ), so potentially could be achieved for conventional systematic reviews if more active communication with trial investigators were adopted. This is seldom done in practice.
Читать дальше













