3 Planning and Initiating an IPD Meta‐Analysis Project
Lesley A. Stewart, Richard D. Riley, and Jayne F. Tierney
IPD meta‐analyses are major research projects that typically take upwards of two years to complete. Specific research funding is usually required, and they cannot be done on a volunteer basis or in spare time. They require broader skills than conventional systematic reviews and meta‐analyses of aggregate data, including greater statistical expertise and experience in managing participant‐level data.
Many IPD meta‐analysis projects are collaborative, involving partnership with trial investigators who contribute IPD from their trials for re‐analysis and whose role is acknowledged through membership of a collaborative group and authorship arrangements. An advisory group may be set up to include wider clinical, topic or methodological expertise and to facilitate patient and public involvement.
Obtaining IPD from repositories or data‐sharing platforms is emerging as a possible alternative or complement to direct collaboration with trial investigators.
Initiating an IPD meta‐analysis requires careful planning and detailed preparation. A clear overview of the entire project is needed at the outset, to give the best chance of success.
Set‐up tasks can include developing a project scope, establishing the central research team and advisory group, seeking in principle agreement to collaborate from trial investigators and/or identifying other sources of IPD, developing data‐sharing agreements, applying for research funding, and for ethical approval or exemption.
A competent central research team should include members with experience of successfully completing an IPD meta‐analysis project, advanced statistical knowledge, experience in managing and coding IPD, and strong communication skills. The team should be as independent as possible and should not include members with a vested interest or strongly held views on the particular topic under investigation, such as whether a particular intervention is or is not effective.
Prospective IPD meta‐analysis projects need detailed and specialist planning.
As described in Chapters 1and 2, IPD meta‐analysis projects are often regarded as a gold standard approach to evidence synthesis. However, they require a broader skillset than conventional systematic reviews, and generally require more resources and take longer to complete. Decisions to embark on IPD projects should therefore not be made lightly, and careful planning is essential. In this chapter, we cover the preparatory phase of an IPD meta‐analysis project and provide guidance for planning and initiation. We outline the importance of carefully refining the research question to be addressed through development of a project scope, building the collaborative framework for the project, and establishing a team equipped with the necessary skills to ensure that it can be delivered successfully. We discuss data‐sharing agreements, outline the potential need for ethical approval, and share our experience of typical timescales and of preparing funding applications. Throughout, we assume that the IPD project aims to synthesise IPD from multiple randomised trials to evaluate treatment effects. However, most of our guidance is also appropriate to other types of IPD meta‐analysis projects including those focused on diagnostic or prognostic topics ( Part 5).
Figure 3.1(phase 1 section) outlines the main activities that need to be completed during the set‐up and initiation phase of an IPD meta‐analysis project, illustrating the approvals that may need to be sought and documentation to be prepared. In order to plan and prepare for an IPD project, and in particular to estimate costs for a funding application, it is necessary to think through all steps of the project at the outset (i.e. all elements of Figure 3.1), as these are often inter‐dependent. Unlike a conventional systematic review and meta‐analysis of existing aggregate data, where if something has been overlooked, syntheses can be unpicked and redone relatively straightforwardly, IPD meta‐analysis projects are not well suited to rapid, frequent or last‐minute changes, because of the considerable additional time that it can take to obtain and check additional data, and re‐run analyses.
3.2 Organisational Approach
3.2.1 Collaborative IPD Meta‐Analysis Project
IPD meta‐analysis projects are most commonly initiated and carried out by a central research team, who establish a wider collaboration including representatives of the included trials. Partnership with trial investigators brings benefits in terms of detailed understanding of the topic area as well as generating ‘buy in’ and ultimately supporting provision of IPD. It also gives trial investigators appropriate academic credit through membership of the collaborative group, in whose name the IPD project is often published. However, direct involvement of trial investigators is at odds with some systematic review standards, such as those produced by the Institute of Medicine which recommend that systematic reviews are undertaken by those who are independent of the primary studies and who do not have vested interest in the review outcome. 73This standard is much easier to comply with when using aggregate data extracted from trial publications. In our view, attempting an IPD meta‐analysis project without some degree of involvement from trial investigators (over and above simply providing or allowing access to their IPD) would significantly reduce the chances of successful completion, and could lead to less insightful and nuanced interpretation of findings. Nonetheless, direct involvement (as a member of the central research team or otherwise having a decision‐making role) of those whose trial is included in the IPD meta‐analysis, or who otherwise hold strong views about the anticipated findings of the research in question, has potential to be both problematic and undermine credibility.
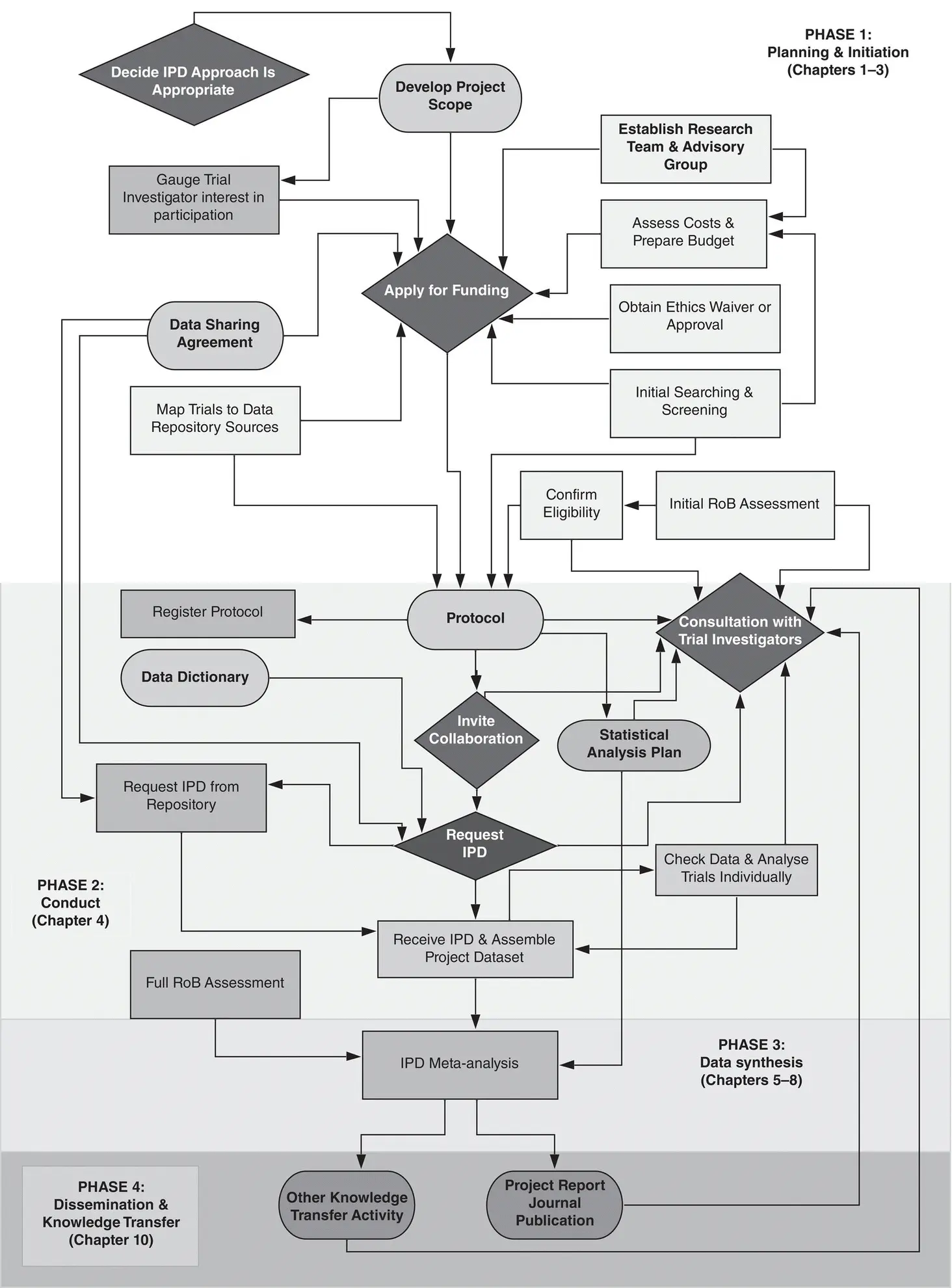
Figure 3.1Typical phases of an IPD meta‐analysis project.
Source: Lesley Stewart.
Therefore, establishing a mechanism that allows trial investigator involvement without permitting vested interest is vital. One approach that may help safeguard against undue influence, whilst still valuing and benefitting from partnership, is for the central research team to have clearly understood responsibility for all aspects of project design and analysis. Whilst the team will listen to advice from both trial investigators and independent advisors (including patients and public stakeholders), and take this into account, they will be responsible for judgements and decisions made. For example, which trials are included, which outcomes are analysed, and which subgroups are examined. Under this framework, the research team should ideally exclude anyone responsible for an included trial. However, it can be difficult to gain sufficient expertise and understanding of the research topic if anyone who has involvement with an eligible trial is precluded from membership of the IPD project’s central research team, particularly for rare health conditions. Thus, a degree of transparent pragmatism may be needed. Certainly, those who have strong beliefs (e.g. about whether an intervention works overall or only in certain individuals, whether particular biomarkers are predictive of treatment effect) or who stand to gain financially or professionally according to the results of synthesis, should not be part of the central team. It is important that the responsibilities of all partners are thought through well in advance, and are made clear to all from the outset of the project, possibly through inclusion in a project scope ( Section 3.3) or charter (and later in the protocol; Section 4.2.2), as well as being reflected in the data‐sharing agreement. An advisory group can also help ensure that the central research team maintains its independence ( Section 3.6).
Читать дальше













